LABORATORY INVESTIGATION: Lab Techniques, Procedures, and
advertisement

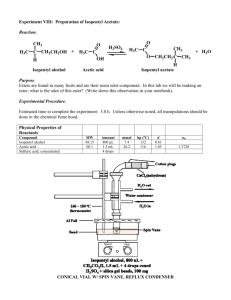
Name: ___________________________________ Block: _________ Date: ___________________ LABORATORY INVESTIGATION: Lab Techniques, Procedures, and Equipment In this lab, you will rotate through several stations, to investigate different lab techniques, procedures, and equipment. At Your Desk: Developing a Lab Methodology - Stoichiometry A large part of organic chemistry is organic synthesis, the construction of organic compounds via organic reactions. Below is the reaction of a carboxylic acid (acetic acid) and an alcohol (isopentyl alcohol) to form an ester (isopentyl acetate or banana oil) and water. O H3C OH Acetic Acid O H2SO4 + H H3C O 3-methyl-1-butanol (isopentyl alcohol) 1 H3CCOOH + 1 C5H11OH H2SO4 + O H OH banana oil (isopentyl acetate) 1 H3CCOOC5H11 + 1 H2O All fragrances and flavorings, natural and synthetic, are volatile organic compounds like isopentyl acetate that selectively bind to and activate olfactory nerves in the nose. Some odors are clearly associated with particular functional groups. Volatile compounds that contain thiol (or sulfide) groups (SH), amine groups (-NH-), or pyridine rings (C5H5N), often have horrible odors. Esters (RCO2R), on the other hand, tend to smell light and fruity. In fact, many fruits owe their pleasant aromas to naturally occurring esters. Some of these ester compounds serve several purposes in nature. Honey bees (Apis mellifera), for example, store isopentyl acetate in their sting gland and emit this compound to signal other members of the hive to sting an enemy. Esters are also commonly found in synthetic flavorings. In a typical experiment to make banana oil, 15 mL of isopentyl alcohol is mixed with 20 mL of acetic acid (liquid) with a small amount of concentrated sulfuric acid. The sulfuric acid serves as a catalyst. 1. Calculate the number of moles of acetic acid in the 15 mL if the density of acetic acid = 1.049 g/mL and its molar mass = 60.05 g/mol # moles = ___________ 2. Calculate the number of moles of isopentyl alcohol in the 20 mL of alcohol if the density = 0.831 g/mL and its molar Mass = 88.15 grams/mol # moles = ___________ 3. What is the limiting reactant if you mix these two reactants together? _____________________ 4. What is the theoretical yield (in grams) of banana oil (isopentyl acetate) from this experiment? Molar Mass of isopentyl acetate = 130.18 grams/mol ______________________ 5. Chemists can use one of the reactants as the solvent in a synthesis; in this case, the acetic acid is used as the solvent and is also a reactant. Explain from the perspective of Le Chatelier’s Principle: Station 1: Making Organic Reactions Go - Chemical Reflux Isopentyl acetate or banana oil (see reaction above in Station 1 section) is a naturally-occurring compound that possesses a distinctive odor. It is found in bananas, artificial banana flavoring, as well as many other organisms. Many organic reactions require heating to increase the reaction rate. For example, to make isopentyl acetate, you need to heat acetic acid and isopentyl alcohol. The apparatus at Station 1 is a typical set-up to heat an organic chemistry reaction. It is called reflux apparatus. Reflux is the process of boiling reactants while continually cooling the vapor returning it back to the flask as a liquid. It is used to heat a mixture for extended periods and at certain temperatures without losing any reactants, solvent, or product through boiling/evaporation. Look at the reflux apparatus and answer the following questions to demonstrate a basic understanding of the process and why it is used: What is the purpose of the condenser? _____________________________________ The synthesis of an ester (like banana oil) from a carboxylic acid and alcohol is endothermic. What two benefits does heating provide (think kinetics and thermodynamics)? Kinetics: ____________________________ Equilibrium: ____________________________ Read the description of the reaction under the previous section and identify other techniques that are utilized to increase the reaction rate in the synthesis of banana oil? _______________________________________________________________________________ What techniques are used to push the equilibrium to the right? ________________________________________________________________________________ ________________________________________________________________________________ Station 2: Isolating and Purifying Products - Separation A separatory funnel (or separation funnel) is a common piece of laboratory glassware used in liquidliquid extractions to separate the components of a mixture into two immiscible solvent phases of different densities. Typically, one of the phases will be aqueous, and the other a non-polar layer made up of a non-polar solvent or product. Non-polar organic solvents are immiscible with water so there is a clear delineation between the two liquids. The two layers formed are usually known as the “organic phase” (non-polar) and the “aqueous phase” (polar). Most of the time, the organic phase is the top layer but not always. A separatory funnel generally consists of a conical or pear-shaped glass body with a stopcock and a stopper on top. The shape allows for the clean separation of the two immisible layers because of a small interface at the bottom. There are some important points that have to be considered. Stopper: It is imperative that it fits tightly so that the solution does not leak out when the separatory funnel is inverted. Stopcock plug: Look at the stopcock plug and understand how it is turned to open and close the funnel. The stopper must be removed when you are draining out a liquid so a vacuum does not build up. Performing a separation/extraction 1. The funnel contains isopentyl acetate with some water-soluble impurities. Your goal is to “wash” the isopentyl acetate with water to extract those water-soluble impurities. 2. Remove the stopper and make sure that the stopcock is closed. 3. Add 25 mL of distilled water. Notice whether the water layer forms on top or on the bottom. 4. Take the separatory funnel out of the ring and hold it tightly at the stopper and the stopcock. Invert it slowly and vent (open the stopcock) pointing the stem of the funnel away from everyone. You will hear a kind of whistle when the pressure is released. 5. Close the stopcock and shake the funnel gently, watching out for emulsions. Vent it again. Repeat this step until no more gas escapes. What does immiscible mean? ______________________________________ What property of a chemical determines whether it will mix with another? ________________ What is happening to any water-soluble impurities when you add water to the funnel and shake? _________________________________________________________________ 6. Place the separatory funnel back in the iron ring. Allow the layers to separate. Then remove the stopper and drain the bottom layer into a clean container. 7. If you are not sure which layer is organic and which one is aqueous, what could you do? _______________________________________________________________________ Station 3: Titration At this station, you will perform a titration to determine the Molarity of an acetic acid solution of an unknown concentration 1. Record the initial volume of solution in each volumetric burette in the Data table below. 2. You need to add about 9.50 mL of the acetic acid solution into a 50 mL Erlenmeyer flask. Based upon your starting volume what should the final volume read on the volumetric pipette. Record this as your goal volume here: _______ Add acetic acid and record the actual final volume in the table (it should be close to your goal volume, but may not be exactly the same) 3. Place 2 drops of phenolphthalein into the flask. 4. Perform a titration to determine the amount of base required to neutralize the acid solution a. The burette contains 0.100 M NaOH b. With the stirring bar spinning, slowly titrate the acid solution with the NaOH solution. c. The phenolphthalein will turn pink when the solution reaches a pH close to 7-8. d. When the pink color first appears slow down your rate of drip. e. When the solution remains pink, then stop your titration and record the final volume of NaOH solution in the burette. You want to try to stop after the one drop that turns it pink. 5. Pour the solution in the waste beaker and rinse the flask out several times. DO NOT LET THE STIR BAR GO DOWN THE DRAIN. Data: Initial Reading ______ mL Acetic Acid _______mL NaOH Final Reading ______ mL Acetic Acid _______mL NaOH Volume Used ______ mL Acetic Acid _______mL NaOH The questions can be completed at your desks when you have completed the lab. Move along to the next station to allow other groups an opportunity to use this station. 1. Determine the volumes used of the acid and base by subtracting the two volumes. 2. When the pH was around 7-8, the acid and base are at the equivalence point and the indicator changed colors. When the indicator changed, you reached the equivalence point, meaning the moles of acid and base are equal. Using the equation MaVa = MbVb determine the molarity of the acetic acid solution. 3. Ask your instructor for the actual molarity of the acid. Determine your percent error using I Experiment – Actual l x 100 % Actual 4. What are some sources of error? Station 4: Common Lab Equipment At this station, you will identify common lab equipment. Identify them by putting the correct letter next to the equipment’s name. ______ Ring ______ Test tube brush ______ Extension clamp ______ Test tube holder ______ Clay triangle ______ Thistle tube ______ Beaker ______ Evaporating dish ______ Test tubes ______ Burette ______ Volumetric flask ______ Plastic wash bottle ______ Graduated cylinder ______ Test tube rack ______ Filter flask ______ Crucible tongs ______ Beaker brush ______ Funnel ______ Wire gauze ______ Rubber stoppers ______ Mortar and pestle ______ Thermometer ______ Watch glass ______ Erlenmeyer flask ______ Bunsen burner ______ Ring stand ______ Crucible with cover ______ Stirring rod Station 5: Boyle’s Law Station 1. Open the volume of the syringe to 20.0 mL and then gently twist it onto the Pressure Sensor. Record the Pressure that is shown on the Lab Quest 2. You will be depressing the volume from 20 to 19 to 18 to … all the way to 10 mL and record the pressure at each 1 mL volume increment. Do NOT go beyond 10 mL as you will break the sensors. 3. With one partner working the syringe, one reading the display and the rest recording the values begin to measure the pressure and volume relationships. 4. Take the inverse of your pressure and record in the data table. 5. Remove the syringe from the pressure sensor and graph your data. a. Label the Y-axis as the Volume and scale the axis from zero to 20 mL b. Label the first X-axis as the Pressure and scale from zero to just beyond your max value c. Label the second X-axis as 1/P and scale from zero to just beyond the max value. Volume Pressure 1/P 20 19 18 17 16 15 14 13 12 11 10 Graph of Volume vs. Pressure Graph of Volume vs. 1/P Station 6 (optional to perform, but complete the questions): Polymers Polyurethane foam is a polymer made from two different monomer components – a polyether polyol and a diisocyanate. The foaming occurs as a result of an additional side reaction that forms carbon dioxide as the diisocyanate reacts with some water. There are many forms of polyurethane including fibers, coatings, flexible foams, and rigid foams. In this experiment, you will form a rigid foam that is similar to the foam used in furniture, packaging, insulation, flotation devices, and many other items. Procedure: Have the teacher tell you how much of “Part A’ to place in one disposable cup. Place an equal amount of “Part B” in a second disposable cup. Add food coloring if you wish to one of the two cups. Combine the two liquids in one of the disposable cups and stir with a wooden stick. Place the cup on paper and watch. DO NOT place either chemical mixture in any glassware! Organic chemicals must contain ____________. The building blocks of a polymer are _______________ Name two natural polymers: _______________________ _______________________________ Name two synthetic polymers (other than polyurethane foam): _________________________ _____________________________
![Isopentyl Acetate [Banana Oil] In this experiment, we prepare an](http://s3.studylib.net/store/data/008730431_1-27d60b2e4c34d7ce18e86c4d6c41e860-300x300.png)