Isopentyl Acetateverbessert
advertisement

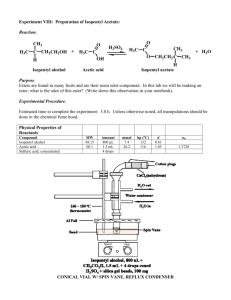
Zurich, 17.11.2007 Synthesis of Isopentyl Acetate O O Tobias Langenegger tobiasla@student.ethz.ch 05-918-362 D-Biol (chem.) assisted by Guo Xiaoqiang Method Esterification of isopentyl alcohol and glacial acetic acid catalysed by sulfuric acid. Chemical equation O O H2SO4 - H2O + OH HO O Mechanism O OH OH H OH OH OH OH HO OH + O HO OH H HO HO OH OH2 O O H H - H2O O O + H O O Physical and safety data substance Mw (g/mol) density (g/cm3) mp (°C) bp (°C) nD20 Poison CH R-phrases S-phrases isopentyl alcohol 88.15 0.81 -117 131 1.406 - 10, 20, 37, 66 46 glacied acetic acid 60.05 1.151 17 117 1.371 3 10, 35 23, 26, 45 isopentyl acetate 98.08 0.876 -78 142 1.400 5 10, 66 23, 25 H2SO4 98.08 1.836 10.4 279.6 - 2 35 26, 30, 45 NaHCO3 84.01 2.22 270 - - - - - NaCl 58.44 2.17 801 - - - - - MgSO4 120.37 2.66 - - - - - - Equipment reflux: distillation: Preparation Substance isopentyl alcohol glacied acetic acid sulfuric acid eq 1 2.537 cat. n (mmol) 68.92 174.85 37.44 V (mL) 7.5 10 2 m (mg) - Experimental section To a solution of 7.5 mL isopentyl alcohol and 10 mL glacial acetic acid, 2 mL concentrated sulfuric acid was added drop by drop, while stirring. This solution then was refluxed for an hour at 140 - 160 °C and after that cooled down to RT. It was washed with water (20 mL), saturated NaHCO3 (3 x 20 mL) and saturated NaCl (20 mL) and dried over MgSO4 over night. The product was purified by distillation (110 – 130 °C) and characterized. Yield V 5.9 mL m 5.1 g n 39.7 mmol Yield 57.6% Characterisation Peaks in IR spectrum nD23.0 = 1.3989 (lit. 1.400) ~2950 cm-1 ~1740 cm-1 ~1230 cm-1 sat. C-H C=O C-O-C Discussion During my distillation, the process stopped and the vapour temperature decreased (while the oil temperature was constant). After a while the temperature increased again and more of the product could be collected. When this happened a second time I stopped the distillation, although there was still some substance. Because this reaction is reversible, we cannot get very high yield. It would be improved, if we added water or alcohol as solvent (big excess). Literature https://www.discoverygate.com/ http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi http://www.chemexper.com/ http://www.wikipedia.org Attachment - Reference IR spectrum Copy of the lab notebook IR spectrum Reference IR spectrum from http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi







![Isopentyl Acetate [Banana Oil] In this experiment, we prepare an](http://s3.studylib.net/store/data/008730431_1-27d60b2e4c34d7ce18e86c4d6c41e860-300x300.png)