Organic Chemistry I Laboratory
advertisement

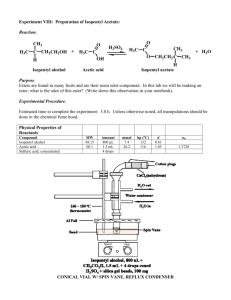
Organic Chemistry I Laboratory Simple Distillation, Gas Chromatography, NMR & Infra-Red Spectroscopy: Experiment 4 Preparation of Synthetic Banana Oil1 Week 4 Background Reading th Zubrick, J. W. The Organic Chem Lab Survival Manual, 4 edition, Wiley & Sons, Inc., New York, 1997. Jointware: Pg 45-55. Boiling stones: Pg 169 Reflux: Pg 227-236 Drying agents: Pg 93-96 Distillation: Pg .193-214 IR Spectroscopy: Pg 313-338 Background Scenario: Your Supervisor has just received the following message from the Cavendish Distilling Company (CDC). Greetings: We have a problem. Cavendish Distilling Company markets the popular liqueur Banana Elixir, which is flavored with a natural banana extract from fruit grown on our Caribbean banana 2 plantation. In June of 1998, Hurricane Floyd blew down all of our banana trees. Our stock of banana extract is running low and our new trees are not yet producing the quantity of fruit needed to supply all of our banana extract needs and we have no alternative source of fresh fruit. As a temporary solution, we have decided to add a synthetic banana flavoring to our remaining stock of the natural extract so it will last until our plantations start producing the quantities of bananas that we need. Synthetic banana flavorings are formulated mainly from isopentyl acetate, with smaller amounts of other esters. I understand that esters can be prepared economically by a process called Fisher esterification which involves the combination of a carboxylic acid such as acetic acid with an alcohol. According to our technical staff, one problem with this method is that Fisher esterifications do not go to completion (<100% yield), leaving considerable amounts of starting material in the products. We can tolerate up to 15% isopentyl alcohol in our isopentyl acetate since the alcohol is a component of natural banana extract. But we cannot tolerate more than 2% acetic acid because it would tend to degrade the flavor of the liqueur. Will you have your consulting chemists prepare some isopentyl acetate and analyze it to see if it falls within our tolerances? Amy Lester, CEO Applying Scientific Methodology: By now you should be familiar with the steps involved in applying scientific methodology to the solution of a problem, so they will not be repeated after this lab. In this experiment the problem described in the scenario does not involve the identity of the product but its purity, so your course of action will include the use of two instrumental methods, gas chromatography and infra-red spectroscopy, to determine the composition of the product. You should be able to gather some evidence regarding the presence of impurities in your isopentyl acetate before you carry out the analysis, so record and evaluate your observations as usual. Esters and Artificial Flavorings The word flavor is used to describe the overall sensory effect of a substance taken into the mouth; flavor may involve tactile, temperature and pain sensations as well as smell and taste. Many fruits flowers and spices contain esters that contribute to their characteristic flavors, where an ester is an organic compound that contains the functional group shown in Figure 1. Most volatile esters have strong pleasant odors that can best be described as “fruity.” Some esters with flavors characteristic of real and “fantasy” fruits are shown in Table 1. The ester you will prepare in this experiment, isopentyl acetate, has a strong banana odor when undiluted and an odor reminescent of pears in dilute solution. It is used as an ingredient in artificial coffee, butterscotch, and honey flavorings as well as in pear and banana flavorings. 1 rd Lehman, J. W. Operational Organic Cheistry, A Problem-Solving Approach to the Laboratory Course, 3 Edition, Prentice-Hall, Inc., Upper Saddle River, New Jersey, 1999, 46-53. 2 The banana plant (Musa cavendishii and other species) is actually a gigantic herb and not a true tree. Each year the foliage-bearing part withers away and is replaced by new growth from an underground stem. Many different esters are included in the basic repertoire of the flavor chemist, who combines natural and synthetic ingredients to prepare artificial flavorings. These ingredients may include natural products, synthetic organic compounds identical to those found in nature, and synthetic compounds not found in nature but accepted as safe for use in food. Each flavor ingredient is characterizes by one or more flavor notes that suggest the predominant impact it makes on the senses of taste and smell. Although the flavor note of a single ingredient may seem unrelated to the overall character of a natural flavor, the combination of carefully selected ingredients in the right proportions can often yield a good approximation of that flavor. A high-boiling fixative such as glycerin or benzyl benzoate is usually added to an artificail flavoring to retard vaporization of volitile components, and the flavor notes of the individual components are blended by dissolving then in a solvent called the vehicle. The most frequently used vehicle is ethanol. O R O R' Figure 1. The Functional Group of an Ester Name Structure Flavor Note O Propyl acetate Pears H3C O O Octyl acetate Oranges H3C O O Benzyl acetate H3C Peaches, Strawberries O O Isopentenyl acetate H3C “Juicy-Fruit” O O Isobutyl propionate H3C Rum O O Ethyl butyrate Pineapples H3C O CH 3 Table 1. Flavor Notes of Some Esters Used in Artificial Flavorings The formulation of artificial flavorings is perhaps as much an art as a science. The components of strawberry flavoring, for example, may vary widely depending on the manufacturer and the specific application. Because natural flavors are usually very complex, a cheap artificial flavoring may be a poor imitation of its natural counterpart, but advances in flavor chemistry have made possible the production of superior flavorings that reproduce natural flavors very closely. Superior flavorings may contain natural oils or extracts that have been fortified with a few synthetic ingredients to enhance the overall effect and replace volitile flavor elements lost during the distillation or extraction process. Even the most experienced flavor chemist can’t hope to do as well as a strawberry plant, which may combine several hundred different flavor components in its berries. But a superior strawberry flavoring with a few dozen ingredients may be hard to distinguish from the real thing, except by the most discriminating of strawberry aficionados. Understanding the Experiment: In this experiment you will prepare synthetic banana oil, which is known by several chemical names including isopentyl acetate, isoamyl acetate, and 3-methylbutyl ethanoate. Esters are often prepared by the Fisher esterification method, which involves heating a carboxylic acid with an alcohol in the presence of an acid catalyst, as shown by the following general equation: O R OH Carboxylic acid + HOR' Alcohol H+ O R OH Ester + H2O The acid catalyst is used to increase the rate of the reaction, which would otherwise require a much longer reaction time. You will synthesize isopentyl acetate by combining isopentyl alcohol (3-methyl-1-butanol) with acetic acid and sulfuric acid and then heating the reaction mixture under reflux for an hour. The alcohol is the limiting reactant, so it should be either weighed or its volume accurately known by using a syringe. The acids can be measured by volume in a less exacting manner since they will be present in excess. The esterification reaction is reversible, having an equilibrium constant of approximately 4.2. If you were to start with equimolar amounts of acetic acid and isopentyl alcohol, only about two thirds of each reactant would be converted into isopentyl acetate by the time equilibrium was reached. Your highest attainable yield in that case would be only 67% of the theoretical 100% yield. To increase the yield of isopentyl acetate you will apply Le Châtelier’s principle, using a 100% excess of acetic acid (2 equivalents). The less expensive reactant, in this case acetic acid, is used to shift the equilibrium toward the products for economical reasons. Even with 2 equivalents of acetic acid, the reaction will not be complete at equilibrium so the product will contain unreacted isopentyl alcohol, the excess acetic acid and the sulfuric acid catalyst. As you learned in experiment 1 this term, a pure component can be obtained from a mixture by separating it from all other components of the mixture using procedures that take advantage of differences in solubility, boiling points, acid-base properties, and other characteristics. Because isopentyl acetate is a liquid, the separation and purification operations will differ from those previously used to separate solid products. At the end of the reflux period, the reaction mixture will contain (in addition to the ester) unreacted acetic acid, sulfuric acid, water, unreacted isopentyl alcohol and some unwanted by-products. Reactions carried out with strong acid catalysts often yield polymeric, tarlike by-products that are high boiling and insoluble in water. Isopentyl acetate is quite insoluble in water wheras both acetic acid and sulfuric acid are water soluble and acidic. This makes it easy to separate the two acids from the product by washing the reaction mixture with water (to remove the bulk of the acids) and then sodium bicarbonate (a weak base) solution. Water will not remove the acids entirely because they are somewhat soluble in the ester as well. The aqueous sodium bicarbonate converts the acids remaing in the ester after water extraction to their sodium salts. These salts are insoluble in the ester but very soluble in water. Therefore the salts migrate to the aqueous layer where they can be separated. The water that forms during the reaction will be separated from the ester along with the wash liquids. Any traces of water which remain are thenremoved by a drying agent (either sodium or magnesium sulfate). Because isopentyl alcohol has a lower boiling point than isopentyl acetate and the by-products have even higher boiling points, it should be possible to remove the alcohol and byproducts from the ester by distillation. Isopentyl alcohol should distill first followed by the ester and any by-products should remain in the distillation flask. The separation is incomplete for reasons described in Zubrick so you will have small amounts of alcohol present in your product ester even after distillation. You will determine the composition of your purified product utilizing both gas chromatography and infra-red spectroscopy. Refer back to experiment 2 and the discussion in Zubrick for an explanation of gas chromatography. Infra-red spectroscopy interacts with bond vibrations. The energy present in the different wavelengths of infra-red light correspond to specific bond vibration modes. Different types of bonds will vibrate at different frequencies. Bonds will be able to absorb light energy of the same frequency as the bond’s fundamental frequency. Therefore, C-H bonds absorb a different frequency of infra-red light than O-H bonds. The -1 characteristic absorbances of the compounds we will be analyzing include the O-H absorption at 3200-3600 cm -1 for alcohols and the carbonyl stretch at 1600-1800 cm . All organic compounds have C-H bonds which absorb at -1 2800-3200 cm . We will use infra-red spectroscopy to determine whether the isopentyl acetate product contains any alcohol impurities. If the ester is pure, there should only be an absorption present for the carbonyl (C=O) group and no O-H stretch observable. Refer to Zubrick for an overview on the technique. One word of caution. Isopentyl acetate is known to be an alarm phermone of the honeybee. Phermones are molecular messangers which produce a response in specific species. When a worker honeybee stings someone it releases a tiny amount (about 1µg) of isopentyl acetate from its stinger. This attracts more honeybees to the scene. Although isopentyl acetate alone does not cause the bees to sting (other phermones in the stinger do that) it agitates them and puts them on guard. Therefore, it might be wise to steer clear of beehives on your way home from lab. Procedure: Under a hood, measure 10 mL of glacial acetic acid and combine it with 75 mmol of isopentyl alcohol in a round bottom flask of appropriate size. Carefully mix in 4-5 drops of concentrated sulfuric acid. Add a couple boiling chips and heat the reaction under reflux for 45-60 min. Separation: When the reaction time is over, allow the mixture to cool nearly to room temperature before you turn off the cooling water and remove the condensor. Transfer the product mixture to a separatory funnel and wash it with 20-25 mL of water, being sure to save the right layer. Carefully wash the organic layer with two successive 15 mL portions of 5% sodium bicarbonate solution, stirring the layers until gas evolution subsides before you stopper the separatory funnel and then venting frequently thereafter. Dry the crude ester with either sodium or magnesium sulfate and carefully decant the crude ester into the disatillation flask and add two boiling chips. Using standard 14/20 glassware, assemble an apparatus for a simple distillation using a “cow” fraction collector. Have the instructor check the set-up before you start heating. Distill the crude product, collecting any liquid that o o distills between 136 C and 143 C. Record the actual boiling range for each fraction which you collect. Weigh your product and take a GC to analyze the purity. Report: From the peak areas of your GC chromatogram, calculate the percentage of each component in the distillate and then determine the percent yield for the overall process. State your conclusion and tell how it is supported by the data. Questions: 1. Calculate the amount of isopentyl acetate that should be present in the product mixture at equilibrium, based on then quantities of starting materials you used and a value of 4.2 for the equilibrium constant. Remember that one molecule of water is formed for each molecule of ester and that concentrations can be expressed in moles since volumes will cancel out. This will simplify the equation you must solve. 2. Estimate the amount of isopentyl acetate (in grams) which was lost as a result of incomplete reaction. 3. What gas escaped during the sodium bicarbonate washing? Write a balanced equation which shows how this gas was formed. 4. In the methodology sectionit was stated that the reaction of an equimolar mixture of isopentyl alcohol and acetic acid will produce at most, 67% of the theoretical amount of isopentyl acetate. Verify this with an equilibrium constant calculation, using K=4.2. Are your experimental results consistent with Le Châtelier’s principle? Explain.