Fischer Esterification
advertisement

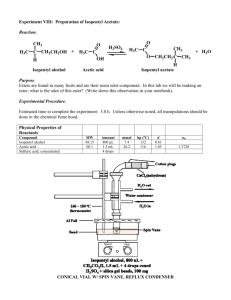
Preparing Isopentyl Acetate by the Fischer Esterification PURPOSE OF THE EXPERIMENT Prepare isopentyl acetate from isopentyl alcohol and acetic acid by the Fischer esterification. BACKGROUND INFORMATION Esters are derivatives of carboxylic acids in which the acyl carbon bears an alkoxy substituent (—OR) rather than the hydroxyl substituent (—OH) of the acid. Simple esters tend to have pleasant, fruity odors and are widely used as flavors and fragrances. Table 1 Composition of the volatile oil of ripe pineapple constituent total volatile oil ethyl acetate ethyl alcohol acetaldehyde ethyl acrylate ethyl i-valerate ethyl u-caproate percent 0.1900 0.1196 0.0605 0.0014 0.0008 0.0004 0.0008 The volatile compounds in natural fruits and flowers are usually complex mixtures of compounds, where esters frequently predominate. Many artificial flavorings contain esters or mixtures of esters. For example, the volatile oil of ripe pineapple contains several esters, as shown in Table 1. Table 2 shows the flavors or fragrances associated with some other esters. Isopentyl acetate is known as banana oil because of its characteristic odor; you will notice the product in this reaction has a strong aroma of banana. This ester has also been shown to be one of the active substances in the alarm pheromone of the honeybee. When a honeybee worker stings an intruder, an alarm pheromone is secreted along with the venom. The pheromone causes other bees to become aggressive and attack the intruder. Esterification generally refers to the formation of esters from alcohols and carboxylic acids, as shown in Equation 1: RCOOH + R'OH RCOOR' + H2O The reaction proceeds by way of a nucleophilic substitution at the acyl carbon of the carboxylic acid. When catalyzed by a strong acid, usually sulfuric acid or p-toluenesulfonic acid, the reaction is called the Fischer esterification. The reaction mechanism is shown in Equations 2 - 6. (I have used general or generic structures here to save space). Table 2 some common esters found in natural fruits and oils. ester fragrance isopentyl acetate isobutyl formate isopentenyl acetate isobutyl proprionate n-propyl acetate methyl butyrate methyl anthranilate methyl salicylate ethyl butyrate ethyl phenyl acetate benzyl acetate banana raspberry juicy fruit rum pear apple grape wintergreen pineapple honey peach 1 Equation 2 shows the protonation of the acyl oxygen of the carboxylic acid. The protonation activates the acyl carbon toward nucleophilic attack. Equation 3 shows the nucleophilic attack at the acyl carbon by the alcohol oxygen atom to form a tetrahedral intermediate. Equation 4 shows a proton transfer to the hydroxyl oxygen of the carboxyl group. This protonation converts the hydroxyl group into the good leaving group, water. Equation 5 shows the loss of water forming the protonated ester. Finally, Equation 6 shows the proton transfer to a base, resulting in the formation of the ester. Protonation (equation 2): + O R OH + H R C C OH OH carboxylic acid protonated carboxylic acid Nucleophilic attack (equation 3): OH + OH R R C C OH O OH + R R1 OH H tetrahedral intermediate Proton Transfer (equation 4): OH R OH C O OH R + C + OH2 O R1 H R1 Dehydration (equation 5): + OH OH R + C OH2 R + C O O R1 H2O R1 protonated ester Deprotonation (equation 6): O + OH R R C + C O O R1 + H R1 2 Each step of the reaction mechanism is reversible and, therefore, the reaction reaches an equilibrium. Le Chatelier’s principle suggests that the amount of ester produced in an equilibrium reaction might he increased either by using an excess of one of the reactants or by removing one of the products. In practice, an excess of carboxylic acid or alcohol, whichever is more readily available, is added, and/or water is removed as the reaction proceeds. A water-absorbing substance such as molecular sieves might he included in the reaction mixture, or the water might be removed as part of an azeotropic with benzene or toluene. The mechanism suggests that steric effects might he important in Equation 3, the step involving the attack by the alcohol at the acyl carbon of the carboxylic acid. Indeed, alkyl-group branching at the or carbon of the acid slows the rate of esterification. For example, the relative rates of esterification with methanol, CH3OH follow the order CH3CO2H > CH3CH2CO2H > (CH3)2CHCO2H > (CH3)3CCO2H Sterically hindered alcohols also react more slowly in the esterification reaction. The relative rates for esterification of alcohols with acetic acid, CH3CO2H, follow the order CH3OH > CH3CH2OH > (CH3)3CCH2OH In this experiment, you will prepare isopentyl acetate by reacting an excess of acetic acid with isopentyl alcohol. After the reaction is complete, you will remove the excess acetic acid from the reaction mixture by extraction with sodium hydrogen carbonate (NaHCO3). You will use sulfuric acid to catalyze the reaction (Equation 7): O + H O + OH HO O + H2O 3 MATERIALS Reagents and Properties_______________________________________________________________ substance quantity molar mass bp d (g/mL) glacial acetic acid isopentyl acetate (product) isopentyl alcohol sodium chloride, saturated solution sodium hydrogen carbonate, 5% sodium sulfate, anhydrous sulfuric acid, concentrated 8.5 mL 4.37 g 60.05 130.19 88.15 142 130 1.049 0.876 0.809 25 mL 50 mL 1.5 g 142.04 1.0 mL 98.08 PROCEDURE Chemical Alert! glacial acetic acid—corrosive isopentyl acetate—flammable and irritant isopentyl alcohol—irritant sodium sulfate—irritant and hygroscopic sulfuric acid—corrosive and oxidant Caution: Wear departmentally approved safety goggles at all times while in the chemistry laboratory! 1. Refluxing the Reaction Mixture Set up a hot plate at your desk and begin heating a 600 mL beaker of water; you will use this for your boiling water bath in the second step. Caution: Isopentyl alcohol (3-methyl-butan-1-ol) is irritating. Glacial acetic acid is corrosive. Concentrated sulfuric acid (H2SO4) is corrosive and oxidizing. Prevent eye, skin, clothing, and combustible material contact. Avoid inhaling vapors and ingesting these compounds. Place 4.37 g (5.4 mL) of isopentyl alcohol into the 125-mL Erlenmeyer flask. Add 8.5 mL of glacial acetic acid and 1 mL of concentrated H2SO4. Add one acid-resistant boiling chips. A glass bead will work well as an acid resistant boiling chip, if available. Cover the opening of the flask with aluminum foil and wrap the foil around the neck of the flask. Heat the reaction in a boiling water bath for 30 minutes, swirling occasionally. The solution does not need to boil during this time, but the water bath needs to be maintained at the boiling point. If the solution begins to boil, reduce the heat. After heating for 30 minutes, turn off the heater and remove the flask from the hot water bath. 4 Note: you may observe a reddish color develop during the beginning of the reaction. This is not the product (isopentyl acetate is clear); this color is probably one of the reactive intermediates of the reaction, possibly the diol cation formed in step 3 of the mechanism. I know from experience that some students will observe this color, and others will not… I’m not sure why!!!! Allow the flask to cool to room temperature. Prepare an ice-water bath in a 400-mL beaker. Cool the flask in the ice-water bath. Place 30 mL of distilled or deionized water in a medium sized test tube; you can use two test tubes if yours are too small. Cool the test tube(s) in the ice-water bath. 2. Separating and Washing the Product Layer Pour 20 mL of the cold water into a 125-mL separatory funnel. Carefully pour the cooled reaction mixture from the Erlenmeyer flask into the funnel containing the water. Be careful not to transfer the glass bead or boiling chip! This will clog the separatory funnel! Rinse the reaction flask with the remaining 10 mL of cold water and pour the rinse water into the separatory funnel, once again taking care to retain the boiling chip in the flask. Swirl the mixture gently, stopper the separatory funnel, and shake once. Vent the funnel carefully. note: Vigorous shaking may result in an emulsion. Label a 150-mL beaker “Aqueous Layers”. Drain the lower aqueous layer from the funnel into the beaker. (It is a good idea to keep all extraction layers in a labeled container until the experiment is finished; this may prevent you from accidentally discarding your product!) Caution: Neutralizing acids with sodium hydrogen carbonate (NaHCO3) generates CO2 gas. Vent the funnel frequently so that pressure does not build up in the stoppered separatory funnel. You should never leave a separatory funnel stoppered for more than 30 seconds or so, particularly in this reaction! Carefully add 25 mL of 5% NaHCO3 to the separatory funnel. Swirl the funnel gently until bubbles no longer appear. Stopper the funnel and shake it cautiously. Vent the funnel. Shake the funnel vigorously and vent it immediately. Return the funnel to the ring stand and immediately remove the stopper. Allow the layers to separate. Remove the stopper and drain the lower aqueous layer into the same labeled beaker. Wash the organic layer in the funnel with a second 25-mL portion of 5% NaHCO3. As before, take care to frequently vent the funnel. Again, drain the lower aqueous layer into the labeled beaker. Finally, wash the organic layer with 25 mL of saturated NaCl. Drain the lower aqueous layer into the labeled beaker. Place about 1.5 g of anhydrous sodium sulfate (Na2SO4) into a 50 mL Erlenmeyer flask. It is not necessary to weigh the sodium sulfate; use about two scoops with your spatula and that will be sufficient. Pour the organic layer into the Erlenmeyer flask. Dry the organic layer over anhydrous Na2SO4 for 5 min. Decant the ester into a clean, dry, pre-weighed container. Record the mass of your product, isopentyl acetate. 5 3. Characterizing the Product Your instructor may provide you with an IR spectrum and/or an NMR spectrum of isopentyl acetate for analysis. 4. Cleaning Up The "aqueous layers" you collected during the lab are pH neutral and non-toxic; they may be washed down the sink with water. Do not discard the isopentyl acetate; although it is non-toxic, your instructor may wish to keep it for further experiments. Clean your glassware by rinsing thoroughly with water; it is not necessary to use soap or detergent. Caution: Wash your hands thoroughly with soap or detergent before leaving the laboratory! 6 Fischer Esterification Lab Report and Post-Lab Name ____________________________________________________________ Post-Lab Questions (please answer on a separate sheet): On a separate sheet, you should provide a data table indicating the masses and/or volumes of chemicals used in the experiment. 1. Based on the quantities of chemicals you used, determine the theoretical yield of isopentyl acetate in grams, and the limiting reactant in this experiment. Calculate your percent yield of isopentyl acetate. You MUST show your work, including calculation of theoretical yield, to receive credit on this question. 2. List the distinctive features of the IR spectra of isopentyl acetate, as well as isopentyl alcohol and acetic acid if provided. Assign the absorption bands to the appropriate functional groups. What are the principal differences between the IR spectra for the reactant and product? (See next pages). 3. Fully analyze the 1H-NMR and 13C-NMR spectra for your product, isopentyl acetate, which are provided on the following pages. Provide a FULL discussion of each spectrum…. indicate where each signal originates, and explain the number of signals, shifts, peak integrations and multiplicities of the signals (as appropriate). For the 1H-NMR spectrum, the best way to do this is to draw the structure of the compound, label each hydrogen type with a letter, and label the corresponding peaks in the spectrum with the same letter (as with many examples in class and in the book). You can do the same thing for the 13C-NMR spectrum, but labeling the carbons instead. 4. a. Write the equation for the reaction of 5% NaHCO3 with acetic acid. b. Why does the reaction mixture release CO2 when it is neutralized with NaHCO3 ? Where does the CO2 come from? 5. What is the IUPAC name for isopentyl acetate? 7 IR spectrum for Isopentyl Alcohol IR spectrum for Isopentyl Acetate 8 1 H-NMR spectrum for Isopentyl Acetate ppm 13 C-NMR spectrum for Isopentyl Acetate 9 Pre-Laboratory Assignment - Fischer Esterification Reaction Name ________________________________________________ 1. What are the hazards you should be aware of when you work with the following reagents? (a) concentrated H2SO4 (b) glacial acetic acid 2. (a) Write a detailed mechanism for the formation of ethyl acetate from ethanol and acetic acid with H2SO4 as catalyst. (b) How does concentrated H2SO4 catalyze the esterification reaction? Briefly explain. 10 3. Describe two methods by which the Fischer esterification equilibrium described in this experiment can be shifted to produce more of the ester. 4. Calculate the limiting reactant, and the theoretical yield of isopentyl acetate (in grams) for the esterification reaction described in the Procedure. Note that the density of glacial acetic acid is provided in the Reagents table as 1.049 g/mL. The overall balanced reaction is shown in Equation 7 at the bottom of page 4. You must show your work to receive credit on this question. (Note: you may also wish to record the result of this calculation, because you will also need it for your post-lab questions). 11