Serum Creatinine Assay
advertisement

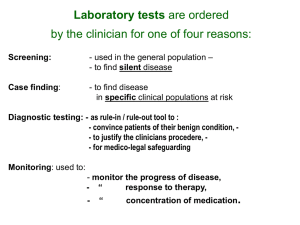
Serum Creatinine Assay for Kidney Function Protocol Id 141401 Version # 1 Description of Protocol This protocol provides instructions for drawing, processing and storing blood for the serum creatinine assay according to the National Health and Nutrition Examination Survey (NHANES) methods. Creatinine concentration in a participant’s serum is measured according to the Jaffe rate method. Specific Instructions The Diabetes Working Group notes that the Serum Creatinine assay can be done in conjunction with Cystatin C assay (see Diabetes Supplemental Information Cystatin C) to yield more information about kidney disease. In particular, comparing and contrasting the polymorphisms associated with each assay can identify "true" markers for kidney function (see Kottgen et al., 2009 for more information). The Sickle Cell Disease Research and Scientific Panel cautions about relying only on the serum creatinine to detect renal insufficiency in sickle cell disease. Because of low muscle mass and an increase in the tubular secretion of creatinine in this disease, a serum creatinine in the normal range does not rule out renal insufficiency (low glomerular filtration rate, GFR) and overestimates the level of GFR. Estimation equations of GFR, widely used in clinical practice, have been derived from non-sickle cell disease populations and lack precision to estimate GFR in sickle cell disease. Based on studies in non-African-American SCD populations, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation without adjustment for ethnicity (Artlet, et al., 2012) may offer the best estimate of GFR in adults, until sickle cell disease specific eGFR equations are developed. Protocol Text The following is a summary version of the full National Health and Nutrition Examination Survey 2007-2008 protocol. Exclusion Criteria Persons will be excluded from this component if they: • Report that they have hemophilia; or • Report that they have received cancer chemotherapy in the last 4 weeks SP= Sample Person. 1. Do you have hemophilia? 1 [] Yes 2 [] No 7 [] Refused 9 [] Don’t Know If the SP answers, "Yes," the SP is excluded from the blood draw. If SP answer "No" or "Don’t Know," blood is drawn from the SP. 2. Have you received cancer chemotherapy in the past four weeks or do you anticipate such therapy in the next four weeks? 1 [] Yes 2 [] No 7 [] Refused 9 [] Don’t Know If the SP answers, "Yes," the SP is excluded from the blood draw. If SP answer "No" or "Don’t Know," blood is drawn from the SP. Note from the Diabetes Working Group: The investigator should record the reason a person is excluded from the blood draw. Venipuncture Procedures Editor’s Note: Please review chapter 4 of the Laboratory Procedures Manual from the National Health and Nutrition Examination Survey for a full description of Phlebotomy procedures. 2007-2008 NHANES Lab Manual. Venipuncture should generally be performed using the median cubital, cephalic, or basilic veins in the left arm unless this arm is unsuitable. If the veins in the left arm are unsuitable, look for suitable veins on the right arm. If the veins in the antecubital space on both arms are not suitable, then look for veins in the forearm or dorsal side of the hand on the left arm/hand and then the right arm/hand. Note from the Diabetes Working Group: Blood should be collected in an appropriate 5- or 10-mL red-top tube. Recording the Results of the Venipuncture Procedure Immediately after completing the venipuncture, record the results of the blood draw, the reasons for a tube not being drawn according to the protocol, and any comments about the venipuncture. Note from the Diabetes Working Group: The Diabetes Working Group recommends that the investigator record whether the blood was drawn and whether the full amount was obtained. Process the Sample for the Serum Creatinine Assay Editor’s Note: Please review chapter 8 of the Laboratory Procedures Manual from the National Health and Nutrition Examination Survey 2007-2008 for a full description of Blood Processing procedures: 2007-2008 NHANES Lab Manual. • Allow the blood to clot by setting aside for 30 to 45 minutes at room temperature. Do not clot for more than an hour. • Centrifuge the tube at room temperature to separate the serum and aliquot into an appropriate storage tube. • Determine if the serum is hemolyzed, turbid, lipemic, or icteric. If so, enter a comment to describe the plasma. Note from the Diabetes Working Group: Serum should be stored at -80°C until testing and shipped on dry ice to prevent thawing. Laboratory Assay for Serum Creatinine The Diabetes Working Group recommends that serum creatinine concentration be determined according to the Jaffe rate method used in the National Health and Nutrition Examination Survey: 2007-2008 NHANES Serum Creatinine Lab Assay. To aid comparability, the Diabetes Working Group recommends that the investigator record the make and manufacturer of equipment used and the repeatability and coefficients of variation for the assay. Reference Ranges* Serum or Plasma mg/dl Age Group Male 0-1 month Female 0.3-0.8 1 month - 1 Year 0.3-0.6 1-15 Years 0.3-1.0 Older than 15 Years 0.7-1.3 0.6-1.1 *From the NHANES protocol for Serum Creatinine Selection Rationale The NHANES 2007-2008 protocol was selected as the best practice methodology and one of the most widely used protocols to measure serum creatinine. Source Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Questionnaire. Laboratory Procedures Manual. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2007. Language English, Spanish Participant Participant 6 years of age or older. Personnel and Training Required Phlebotomist Laboratory that can perform the Jaffe rate method Equipment Needs Phlebotomy supplies Standards General References Standard Name ID Source Common Data Elements (CDE) Hematology Serum Creatinine Laboratory Result Value in mg/dL 2655822 CDE Browser Logical Observation Identifiers Names and Codes (LOINC) Serum creatinine assay proto 62807-3 LOINC American Diabetes Association. (2010). Diagnosis and classification of diabetes mellitus. Diabetes Care, 33 (Supplement 1), S11 - S61. Kottgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, Yang Q, Gudnason V, Launer LJ, Harris TB, Smith AV, Arking DE, Astor BC, Boerwinkle E, Ehret GB, Ruczinski I, Scharpf RB, Ida Chen YD, de Boer IH, Haritunians T, Lumley T, Sarnak M, Siscovick D, Benjamin EJ, Levy D, Upadhyay A, Aulchenko YS, Hofman A, Rivadeneira F, Uitterlinden AG, van Duijn CM, Chasman DI, Par�, Ridker PM, Kao WH, Witteman JC, Coresh J, Shlipak MG, & Fox CS. (2009). Multiple loci associated with indices of renal function and chronic kidney disease. Nature Genetics. 41(6), 712-717 Allon, M., Lawson, L., Eckman, J.R., Delaney, V., & Bourke, E. (1988). Effects of nonsteroidal antiinflammatory drugs on renal function in sickle cell anemia. Kidney International, 34, 500-506. Arlet, J.B., Ribeil, J.A., Chatellier, G., Eladari, D., De Seigneux, S., Souberbielle, J.C., Friedlander, G., de Montalembert, M., Pouchot, J., Pri�D., & Courbebaisse, M. (2012) Determination of the best method to estimate glomerular filtration rate from serum creatinine in adult patient with sickle cell disease: a prospective observational cohort study. BMC Nephrology, 13, 83 de Jong, P.E., de Jong-Van Den Berg, T.W., Sewrajsingh, G.S., Schouten, H., Donker, A.J.M., & Statius van Eps, L.W. (1980). The influence of indomethacin on renal hemodynamics in sickle cell anemia. Clinical Science, 59, 245-250. Guasch, A., Cua, M., & Mitch, W.E. (1996). Early detection and the course of glomerular injury in patients with sickle cell anemia. Kidney International, 49, 786-791. Protocol Type Bioassay Derived Variables Estimated Glomerular Filtration Rate (eGFR): For Creatinine in mg/dL: Estimated Glomerular Filtration Rate = 186 X Serum Creatinine Age -.203 X [1.210 if African American] X [0.742 if Female] -1.154 X For Creatinine in umol/L: Estimated Glomerular Filtration Rate = 32788 X Serum Creatinine Age -.203 X [1.210 if African American] X [0.742 if Female] -1.154 X Levey, AS, Bosch, JP, Lewis, JB, Greene, T, Rogers, N, & Roth D. (1999). A more accurate mehtod to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of Internal Medicine. 130(6), 461-470. Requirements Requirement Category Required Average time of greater than 15 minutes in an unaffected individual No Average time of greater than 15 minutes in an unaffected individual Major equipment This measure requires a specialized measurement device that may not be readily available in every setting where genome wide No association studies are being conducted. Examples of specialized equipment are DEXA, Echocardiography, and Spirometry Specialized requirements for biospecimen collection No This protocol requires that blood, urine, etc. be collected from the study participants. Specialized training This measure requires staff training in the protocol methodology and/or in the conduct of the data analysis. No