Quiz 1 - Sites@UCI

1. Choose 2 of each of these questions per section (do not use the first two together since they both ask about acid/bases)
Circle the functional group present in fatty acids –(carboxyl) C E
Circle the functional group that can act as a weak base –(amino) D
Circle a functional group commonly found in monosaccharides (OH or carbonyl) E D
Circle the functional groups found in the backbone chain of a protein (amino and carboxyl) C
Circle the functional group that, when present in the side chain of a protein, stabilizes the terciary structure by forming covalent bonds (SH) D
Circle a functional group that can be found in steroid sex hormones (methyl or hydroxyl) E
Circle a functional group that can be present in phospholipids (phosphate) C
2. Choose one for each section – mix the options for each question too --
Polymers of amino acids are disassembled by:
A) the formation of phosphodiester linkages
B) the addition of hydrogen ions to each monomer
C) hydrolysis (correct)
D) ionic bonding of the monomers
E) the formation of disulfide bridges between monomers
Which of these cannot be a subunits of a polymer:
A) a monosaccaride
B) a nucleotide
C) a choline group
D) an amino acid
E) A and B
Quaternary protein structures are stabilized by interactions between
A) carboxyl groups
B) amino groups
C) side chains (R groups) (correct)
D) primary structure
E) backbone chain strucutre
3. Choose one for each section --
Write a short sentence about one important function of proteins –
Write a short sentence about one important function of polysaccharides –
Write a short sentence about one important function of lipids –
Write a short sentence about one important function of DNA --
4. Choose one for each section –
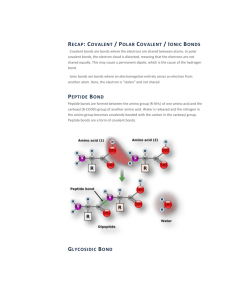
What type of bond is a glycosidic linkage? In what type of polymers is it present?(covalent / polysaccharides, sugars, carbohydrates)
What type of bond is a peptide bond? In what type of polymers is it present? (covalent / polypeptides, proteins)
What type of bond is the phosphodiester linkage? In what type of polymers is it present?
(covalent / nucleic acids, DNA, RNA)
What type of bond is the ester linkage? In what type of molecule is it present?
(covalent / lipids, fats)
5. Use these choices to answer the following questions.
A. nonpolar covalent bonds…
B. polar covalent bonds…
C. ionic bond…
D. hydrogen bonds…
E. hydrophobic interaction…
F. hydrophobic cohesion…
I) …are common between metal and non metal elements. (ionic bond)
II) …are common between carbon and other atoms like O, N or H. (nonpolar covalent)
III) …are responsible for the capacity of water to move along plant vessels against gravity
(hydrogen bonds)
Use only two questions per section and mix them up
6. – choose one for each discussion section –
A. Which of the following solutions has the lowest concentration of hydroxide ions [HO
-
]?
A) gastric juice at pH 2 (correct)
B) vinegar at pH 3
C) tomato juice at pH 4
D) black coffee at pH 5
E) household bleach at pH 12
B. Which of the following solutions has the highest concentration of hydroxide ions [HO
-
]?
A) vinegar at pH 3
B) lemon juice at pH 2
C) tomato juice at pH 4
D) urine at pH 6
E) seawater at pH 8 (correct)