Review for test
advertisement

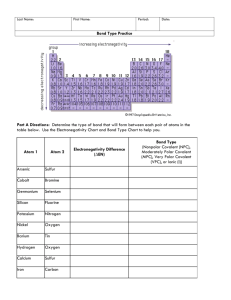
Covalent Bond Test Review Vocabulary: Single Covalent Bond Double Covalent Bond Triple Covalent Bond Unpaired Shares Structural Formulas VSEPR Theory Dispersion Forces Polar Covalent Bonds Nonpolar Covalent Polar Bond Polar Molecule Network Solid Coordinate Covalent Bond 1) 2) 3) 4) 5) 6) 7) 8) How do you identify a covalent vs. an ionic compound? What are characteristics of covalent bonds? What is a diatomic molecule? Which elements are found in nature as diatomic molecules? Name the following compounds. a. SCl2 b. P2S5 c. NBr3 Write the chemical formula for the following: a. Silicon tetrabromide b. Dinitrogen trioxide c. Sulfur hexachloride Draw the Lewis Structure for the following: a. PH3 b. H2S c. SO3 d. CCl4 Determine if the following compounds are polar or non-polar. a. I2 b. CO2 c. N2O d. PF3 Using the chart on pg. 405 of your book, determine if the following bonds are polar, nonpolar, or ionic. a. H and F b. Mg and O c. N and N d. C and S e. N and P