Abstract This project was a proof-of
advertisement

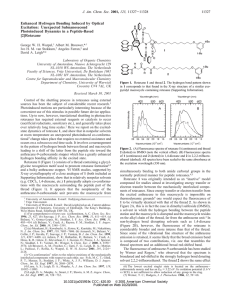
Abstract This project was a proof-of-concept for a new synthetic strategy towards rotaxanes, obviating the need for a recognition pattern between the ring and thread fragments. A scaffold, which can be removed later on, is used to accomplish the pre-organization of the components. If this proof-of-concept will work, it should open the way towards rotaxanes which cannot be made via conventional methods, like natural peptide rotaxanes such as Microcin J25. During this project the ring- and thread fragments 1 and 2 (see figure 1) were designed to incorporate multiple functionalities (which are needed in the strategy), as well as being symmetric (therefore being available via a convergent synthesis and also aiding analysis). They also contain peptide-mimicking structures, thereby already testing whether it is viable to make peptide rotaxanes. For the sake of simplicity, no chirality is involved in this project. The strategy how to make a rotaxane using the functionalities is outlined in the introduction (goal of the project). Figure 1: Ring and thread fragments During the project, the original design of a hydroxylamine functionality directly at the phenyl group (1 and 2) gave problems during reductive amination (a crucial step in the synthesis), therefore an ethylene spacer was placed in between those moieties. Now, ring- and thread fragments 3 and 4 were both accessible, although neither could be obtained pure due to difficult chromatographic removal of impurities. Moreover, due to hindered rotation of the central carbamate moiety, NMR analysis was complicated for both fragments. Despite various affords to synthesize a scaffold which can facilitate the pre-organization of the ringand thread fragment, no synthesis route so far has been successful. This remains the biggest synthetic challenge of the project now.