CH115-1C Patterson SI Worksheet 8 For the following, draw the
advertisement

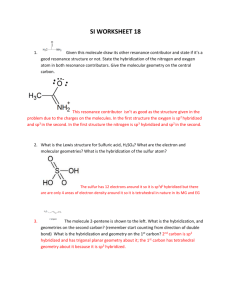
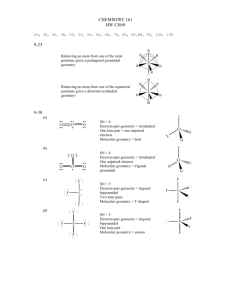
CH115-1C Patterson SI Worksheet 8 For the following, draw the molecule and list the name or the chemical formula, the number of regions of electron density, the electronic geometry, the molecular geometry and the hybridization about the central atom. *Remember to use dashed and wedged lines if necessary -- #s 1, 3, 5, and 6 will be best solved by trying to find the formal charge of the atoms and deciding which configuration is more stable 1. Dihydrogen Sulfate (Sulfuric Acid) H2SO4 2. Xenon Difluoride XeF2 Regions of Electron Density: 5 Electronic Geometry: trigonal/ triangular pyramidal Regions of Electron Density: 4 Molecular Geometry: linear Electronic Geometry: tetrahedral Hybridization of central atom: sp3d Molecular Geometry: tetrahedral Hybridization of central atom: sp3 3. Permanganate MnO4- 4. Hydrogen Acetate (choose one carbon) HCH3COO (this can be written many ways) Stereochemistry is not shown Stereochemistry is not shown Regions of Electron Density: 1. 4 / 2. 3 Electronic Geometry: 1. tetrahedral / 2. Trigonal planar Regions of Electron Density: 4 Electronic Geometry: tetrahedral Molecular Geometry: tetrahedral 3 Hybridization of central atom: sp Molecular Geometry: 1. tetrahedral / 2. Trigonal planar Hybridization of central atom: 1. sp3 / 2. sp2 CH115-1C Patterson SI 5. ClO3- Worksheet 8 Chlorate ion 6. N2O4 Dinitrogen tetroxide Regions of Electron Density: 4 Regions of Electron Density: 3 Electronic Geometry: tetrahedral Electronic Geometry: trigonal planar Molecular Geometry: triangular pyramidal Molecular Geometry: trigonal planar Hybridization of central atom: sp3 Hybridization of central atom: sp2 7. Dihydrogen Monoxide H H2O 8. S2O32- Thiosulfate ion O H Regions of Electron Density: 4 Electronic Geometry: tetrahedral Regions of Electron Density: 4 Molecular Geometry: tetrahedral Electronic Geometry: tetrahedral Hybridization of central atom: sp3 Molecular Geometry: tetrahedral Hybridization of central atom: sp3 9. IF5 Iodide pentafluoride 10. TeCl4 Cl F F F I F Tellurium tetrachloride F Cl Te Cl Cl Regions of Electron Density: 6 Regions of Electron Density: 5 Electronic Geometry: octahedral Electronic Geometry: trigonal bipyramidal Molecular Geometry: square pyramidal Molecular Geometry: seesaw Hybridization of central atom: sp3d2 Hybridization of central atom: sp3d CH115-1C Patterson SI 11. Worksheet 8 Chlorine Tribromide ClBr3 12. CO2 carbon dioxide Br Cl Br Br Regions of Electron Density: 2 Electronic Geometry: linear Regions of Electron Density: 5 Molecular Geometry: linear Electronic Geometry: trigonal bipyramidal Hybridization of central atom: sp Molecular Geometry: T-shaped Hybridization of central atom: sp3d ***Bonus: Can you state the bond angles that you should find for all of the molecules above? 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 109.5 90, 120 109.5 109.5 / 120 109.5 120 109.5 (104.5) 109.5 90 90, 120 90, 120 180