CHEMISTRY 161 - Seattle Central College
advertisement

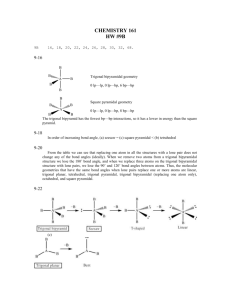
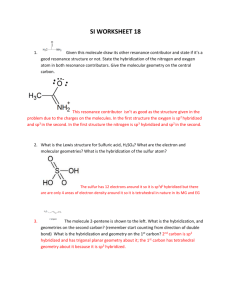
CHEMISTRY 161 HW CH#9 23, 30, 36, 38, 50, 52, 56, 60, 68, 76, 82, 86,88, 90, 100, 108 9-23 B Removing an atom from one of the axial positions gives a pentagonal pyramidal geometry B B A B B B B Removing an atom from one of the equatorial positions gives a distorted octahedral geometry B B A B B B 9-30 (a) SN = 4 Electron-pair geometry = tetrahedral One lone pair + one unpaired electron Molecular geometry = bent (b) SN = 4 Electron-pair geometry = tetrahedral One unpaired electron Molecular geometry = trigonal pyramidal (c) F F F SN = 5 Electron-pair geometry = trigonal bipyramidal Two lone pairs Molecular geometry = T-shaped I F (d) S F I F F F F F F SN = 5 Electron-pair geometry = trigonal bipyramidal One lone pair Molecular geometry = seesaw S F F F 9-38 H Xe S SN = 5 Electron-pair geometry = trigonal bipyramidal Three lone pairs Molecular geometry = linear around Xe H 9-50 (a) Nitrogen is more electronegative than P so NH 3 has the most polar bonds and, therefore, is more polar than PH3. (b) Bromine is less electronegative than Cl. In CBr2F2 the Br atoms do not counteract the electron pull from the F atoms as well as Cl does, so CBr2F2 is more polar than CCl2F2. 9-52 From the periodic table we would estimate the atoms increase in electronegativity in the order Si < S < C < N < O. The greater the electronegativity difference between the atoms, the more polar the diatomic molecule. Compounds made up of elements in the list that are far from each other (e.g., Si and O) have the largest dipole moment because they are the most polar. Molecules made up of elements closest together in the series have small differences in electronegativity and so are less polar with lower dipole moments. We might guess an order of polarities, then, as SiS < CS < NO < CO < SO < SiO. Using the electronegativity values from Figure 8.5, we obtain the molecules in order of increasing dipole moment of CS < NO < SiS < SO = CO < SiO. 9-56 + (a) O N O (b) O N O SN = 2 sp hybridized SN = 3 sp2 hybridized SN = 2 for both N atoms sp hybridized SN = 3 for both N atoms sp2 hybridized SN = 3 for both N atoms sp2 hybridized – (c) O (d) O O N N O O O (e) N N O O 9-60 F F S SN = 4 sp3 F F F F S F SN = 5 sp3d F F S F F F SN = 6 sp3d 2 9-68 Because the steric number, and thus the geometry, around each central atom in a larger molecule must be defined separately and may be different for adjacent atoms, the overall molecular geometry is sometimes hard to name. For example, if a tetrahedral atom is bonded to a trigonal planar atom, just one term cannot describe the geometry of the molecule X Y A X X B Y 9-76 The S atoms have SN = 4 and are tetrahedral. The central O atom also has SN = 4 and is bent with sp3 hybridization. 9-82 Molecular orbital theory better explains magnetic properties because the orbitals are placed in terms of energy, like atomic orbitals. If there are unpaired electrons in any molecular orbital energy level, the molecule will be attracted to a magnetic field. 9-86 Overlap of an s orbital with either p or d orbitals side-on will result in no net overlap because of the different phases of the lobes on the p and d orbitals. Overlap of a p orbital with a d orbital gives constructive interference, but the overlap is not too effective because the p and d orbitals are of different energies. Overlap of a 3p orbital with a 3p orbital, or 3s with 3s, or 3d with 3d, gives constructive interference with efficient overlap because of their similar energies and shapes. 9-88 9-90 He2 would have the MOs 1s 1*s Ne2 would have the MOs 2s 2*s 2 p 2 p 2* p 2* p The bond order (BO) is calculated from BO = 12 (number of e– in bonding MOs– – number of e– in antibonding MOs) Solve + He 2 Total number of electrons = 3 e ( 1s ) 2 ( 1*s )1 BO = 12 (2 1) = 0.5 Ne 2 + total number of electrons = 15 e ( 2 s ) 2 ( 2*s ) 2 ( 2 p ) 2 ( 2 p ) 4 ( 2* p ) 4 ( 2* p )1 BO = 12 (8 7) = 0.5 Each of these species has a bond order of and Ne2. 1 2 , so each would be predicted to be more stable than He2 9-100 In this Lewis structure all the negative formal charges are on the most electronegative atoms (oxygen) SN = 4 Electron-pair geometry = tetrahedral Molecular geometry = tetrahedral Bond angles = 109.5˚ 9-108 F Al SN = 3 with 3 bonds to Al Molecular geometry = trigonal planar sp2 hybridization F F – F F Al SN = 4 with 4 bonds to Al Molecular geometry = tetrahedral sp3 hybridization F F 2– F F Al F F SN = 5 with 5 bonds to Al Molecular geometry = trigonal bipyramidal sp3d hybridization F SN = 6 with 6 bonds to Al Molecular geometry = octahedral sp3d 2 hybridization