SCH3Ulabunit3
advertisement

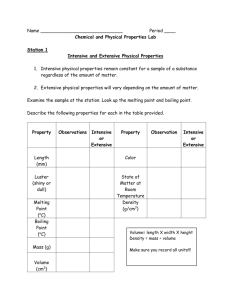
SCH3U UNIT 3 Labs Quantities in Chemical Reactions Complete the following dry labs on your own in your lab notebooks. Determination of Relative Atomic Mass Pg 166, 167 Q: a-g Designing and Testing Gravimetric Stoichiometry: Calculating an Excess Reagent Pg 232, 233 Q: a-f Complete the following investigations and record all results and analysis questions in your lab book. Percentage Composition by Mass of Magnesium Oxide Question: What is the percentage composition by mass of magnesium oxide? Is this percentage constant? Experimental Design: A known mass of magnesium metal is heated in a crucible over a laboratory burner. The mass of the magnesium oxide formed is used to determine the mass of the oxygen that reacted and the percentage composition by mass of the two components. Some of the magnesium also reacts with nitrogen in air to form a nitride. This compound is converted to magnesium oxide by adding water and reheating the solid. Materials: 7-8 cm magnesium ribbon, steel wool, porcelain crucible and lid, burner, retort stand, ring stand and clamp, wire, crucible tongs, glass stirring rod, distilled water Analysis: 1. What evidence do you have that a chemical reaction took place? 2. Calculate the mass of oxygen that reacted with the magnesium 3. Use your evidence to calculate the percentage composition by mass of magnesium oxide 4. Answer the lab question 5. If some of the magnesium oxide had escaped from the crucible, would your percentage composition calculation of magnesium be too high or too low? Explain. 6. If the magnesium had reacted with some other component in the air, would the percentage composition calculation of magnesium be too high or too low? Explain 7. The magnesium ribbon was polished to remove any white film on its surface before beginning the experiment. Explain why this is necessary. Determining the Formula of an Unknown Hydrate Question: What is the chemical formula of copper (II) sulfate hydrate? Experimental Design: A known mass of a hydrate of copper (II) sulfate is heated until all water of hydration has been removed. The mass of water of hydration is calculated and evidence is analyzed to determine the amount in moles of hydrate and water of hydration. Materials: 4 g of copper (II) sulfate hydrate Crucible and lid, tongs, wire, iron ring and stand, burner, and balance Analysis: 1. Calculate the percent by mass of water in your sample of hydrated copper (II) sulfate. 2. Based on your observations, determine the chemical formula for CuSO4· xH2O (s). 3. Suppose that you heated a sample of a hydrated ionic compound in a test tube. What might you expect to see inside, near the mouth of the test tube? Explain. 4. Using your observations, calculate the percentage composition of the hydrated copper (II) sulfate 5. Suppose that you did not completely convert the hydrate to the anhydrous compound. Explain how this would affect the calculated percent by mass of water in the compound, and the chemical formula you determined. Quantitative Analysis of Sodium Carbonate Solution Question: What is the mass of sodium carbonate present in a 50.0 mL sample of a solution? Experimental Design: The mass of sodium carbonate present in the sample solution is determined by having it react with an excess of calcium chloride in solution. A double displacement reaction takes place; one of the products formed, calcium carbonate, is insoluble in water. The only other product formed is sodium chloride, which is soluble in water. The mass of calcium carbonate precipitate produced is used to determine the mass of sodium carbonate reacted. The value is read from a standard curve plotted using reference data in Table 2 (pg 206). Materials: 0.5 mol/L (use 50.0 mL) sodium carbonate, 0.3 mol/L (use 100.0 mL) calcium chloride, distilled water, 100 mL graduated cylinder, 2 50 mL beakers, stirring rod, dropper, filter paper, funnel, balance Analysis: 1. What is the mass of calcium carbonate produced in your sample? 2. What is the shape of the standard curve plotted with the reference data? What type of relationship exists between the mass of reactant and mass of product in this reaction? 3. From the standard curve, what was the mass of sodium carbonate in your sample? 4. Suppose the precipitate formed in a similar reaction is slightly soluble in water. Can a standard curve still be used to determine the mass of reactant? Which Reagent is Limiting and how much Precipitate is Formed? Question: What is the mass of precipitate produced by the reaction of 2.00 g of strontium chloride with 2.00 g of copper (II) sulfate pentahydrate in 75 mL of water? Experimental Design: Strontium chloride reacts with copper (II) sulfate pentahydrate to produce strontium sulfate as a precipitate. The other product formed is copper (II) chloride, which remains in solution. Analysis: 1. Answer the lab question Finding the Percentage Yield of a Single Displacement Reaction Question: What is the percentage yield of the precipitate in the reaction of iron and copper (II) chloride when steel wool and aqueous copper (II) chloride are used as reactants? Experimental Design: The percentage yield of any reaction can be affected by several factors. One of these factors is the impurity of the reactants. In this investigation, you will conduct a single displacement reaction in which the iron in steel wool reacts with aqueous copper (II) chloride to produce aqueous iron (II) chloride and a precipitate. You will then determine the percentage yield of the precipitate in the reaction and determine the effect of an impure reactant on this yield. Materials: 5.0 g of copper (II) chloride dehydrate, Distilled water, 1.00 g of steel wool, 10 mL of 1.0 mol/L HCl, 2 250 mL beakers, Balance, Stirring rod Analysis: 1. Identify the precipitate that was formed in the reaction. 2. Which reactant was the limiting reactant and which was excess? 3. Calculate the theoretical yield of the precipitate in grams, using the mass of the steel wool that you used. 4. Compare the actual yield to the experimental yield. 5. Calculate the percentage yield of this reaction. 6. Suggest reasons why the percentage yield of this reaction was not 100 % 7. Explain how the impurity of the iron affected the actual yield