Activity 43
advertisement

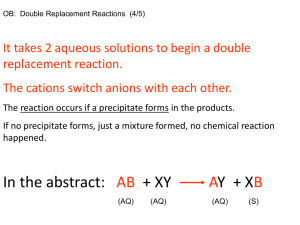
Major Concepts Activity 40 • The concentration of a solution is expressed in terms of parts of solute to parts of solution. • Parts per million (ppm) are used to express concentrations with very small solute to solution ratios • Concentrations can be expressed as parts per ___, fractions, ratios, % Activity 45 Follow-up Precipitation and Filtration Results • Test 1: Not all contaminant has precipitated because there is blue solution remaining around the edges of the precipitate. • Test 2: Not all contaminant has precipitated, but more than Test 1. • Test 3: Not all contaminant has precipitated, but more than Test 2. • Test 4: All of the contaminant has precipitated because there is no blue solution remaining; there is only solid blue precipitate present. Activity 45 Analysis 1. What was the contaminant in this activity? – Copper chloride 2. What evidence indicates that a chemical reaction occurred when you mixed solutions of sodium carbonate and copper chloride? – Color change – Appearance of new solid – Bubbles (release of gas) 3. a. You added sodium carbonate solution to the copper chloride solution. Where do you think the solid that appeared came from? – A chemical reaction between the sodium carbonate and copper chloride – It’s called a precipitate b. Why does the substance get trapped by the filter? – It is an undissolved solid and is too large to pass through the filter c. What property(ies) does (do) all solid precipitates that form and settle to the bottom when two solutions are mixed have? – All solid precipitates are slightly soluble – They appear as solids and do not remain dissolved – Since the solids settle to the bottom, they must be more dense than the surrounding liquid. 4. Describe two ways the control in Test 1 helped you analyze the data. – Used as a comparison to the other 3 tests – Helped determine if all the contaminant had precipitated out – Helped in comparison after filtering and the procedure on the filtrate to make sure that contaminant was detected in it 5. a. Did precipitation work for removing the contaminant from the water? Explain, using evidence from the investigation. – Precipitation worked for removing the contaminant in Test 4 – The filtrate looked clear and colorless and there was a lot of precipitate on the filter – Test 1 had the most remaining contaminant because the filtrate was very blue and there was no precipitate in the filter paper – Tests 2 and 3 were in between 5. b. Did your procedure for testing the presence of contaminant in the filtrate work well? How did you know how well it worked? – Yes, a precipitate formed when sodium carbonate was added – No, no precipitate formed when sodium carbonate was added 5. c. If your procedure did not work well, think of at least one way you could improve it. – Add more drops of sodium carbonate – Do an ammonia test – Do both tests 6. How could the procedure in this investigation be useful for purifying wastewater? – Chemicals that react with the dissolved substances to form a precipitate can be added to the waste water to form a precipitate – The precipitate can be filtered out 7. Copper is a metal. Look at the Periodic Table of Element, and list two other elements that you think this procedure would work well for if they were contaminants. – Any metal near copper including cobalt, iron, nickel, silver, etc. Activity 43 Title: Municipal Water Treatment Read C-67 Problem: How do community water districts ensure that the water they provide is safe? Hypothesis/Initial Thoughts: Stopping to Think (STT) • Do #1, 2c, 4a and 4b only • Do Analysis 1-5 • The book is available on my web page if you do not finish