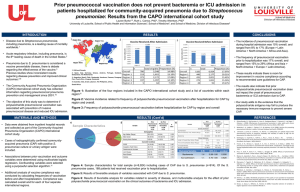
Studies assessing the effectiveness of routine use vaccination with
advertisement

Studies assessing the effectiveness of routine use vaccination with pneumococcal conjugate vaccines on pneumonia prevention Country Schedule Observation Data source period Case detection Canada1 3+1 1989-2001 ICD-9 Australia2 3+0 1998--2007 US3 3+1 US4 3+1 US5 3+1 1997-2004 US6 3+1 ICD-9-CM US7 3+1 1997-1999 vs National Inpatient Sample 2001-2004 database 1998-2004 Group Health (Washington state) database Australia8 3+PPS ICD-9, ICD-10 AM Australia9 3+PPS 1996-2000 Western Australia Data vs 2001-2005 Linkage System, indigenous population 1998-2005 Historical indigenous cohort Italy10 2+1 US11 3+1 Med-Echo administrative database Australian Institute of Health ICD-10-AM and Welfare National Hospital Morbidity Database 1994-2003 National Ambulatory Medical ICD-9-CM Care Survey, National Hospital Ambulatory Medical Care Survey 1996-1999 vs Nationwide Inpatient Sample ICD-9-CM 2001-2007 and Census Market Scan Database 2000-2002 vs Regional database 2003-2005 1997 vs 2006 Kids’ Inpatient Database ICD-9 % reduction in pneumonia outcome [95% CI] All cause pneumonia (age in years) Pneumococcal pneumonia (age in years) Ambulatory visits Hospital Radiological Complicated Ambulatory Hospital Radiological discharges pneumonia pneumonia visits discharges pneumonia Empyema: No trend 13·2% (<5) 72·3 (<5) (Lobar) observed (0·8/100,000 population <5) 38% [36-40](<2) 28% [21-34] (2-4) 31% [-23-85]† (<2) 33% (<2) +0·5% (2-4) 41·1% [40-42] (<2) ICD-9-CM 61%[(<2) 26% (2-4) 46·9% [35- 57·6% [41-75] 58] (<2) (<2) 65% [47-77] (<2) 73% [53-85](2-4) 52·4% [48-53] (<2) 39% [22-52] (<2) 17% [-3-34] (2-4) ICD-9-CM Retrospective CXR review ICD-9-CM Empyema: +100% 3·5 to 7·0 per 100,000 population (<2). +180% 3·7 to 10·3 per 100,000 (24) Inpatient 40% [-4- 65] (<1) Outpatient 36% [5-42](<1) No change (1-4)** 34% (1-2) 44% (2-6) 24% [-9-47] (>518m) 15·2% [2·8-26·1] (<2) 29% (<2) ║ 70·5 [9·7-90·4] (<2) Empyema +100% (3·8 to 7·6 per 100,000 1 population, <5) +131% (3·1 to 8·1 per 100,000 population, 2-4) US12 3+1 1996-1999 vs Health Care Utilization Project ICD-9 2005-2006 State Inpatient Database 28% [27-30] (<2) 1% [-1-4] (2-4) US13 3+1 1997 vs 2006 Kids’ Inpatient Database ICD-9-CM 21·9% (<1) +1·9 (1-5) US14 3+1 ICD-9-CM 35% (<2) 0% [-1-1](2-4) US15 3+1 UK16 2+1 1997-1999 vs National Inpatient Sample 2005-2006 database 1994-2007 National Ambulatory, National Hospital Ambulatory Medical Care Survey 1997-2006 vs Hospital Episodes Statistics 2007-2008 database Poland17 2+1 Uruguay18 2+1 2004-2008 ICD-9-CM ICD-10 Polyclinic children hospital ICD-10 database/ National Heath Fund 2005-2007 vs Reports of Disease of not stated 2009 Mandatory Reporting (Ministry of Public Health), HP-CHRP database, HP-CHRP Microbiology Laboratories Database 47% [38-54] (<2)* 12 [-2-24](2-4) Any 25·5% (54·9 to 40·9 per 100,000 population, <1) +31·5 (19·7 to 25·9 per 100,000 population, 1-5) Local + 67·2% (6·7 to 11·2 per 100,000 population, <1) +79·3% (9·2 to 16·5 per 100,000 population, 1-5) Systemic 35·5% (49·0 to 31·6 per 100,000 population, <1). No change (1-5) No change (1-5) p=0·96 19 %(<15)║ Empyema 22% (18 to 14 per million population, <15) 65% (<2) 23% (2-5) 56% (<14) Empyema 35% (106 to 69 per 10,000 discharges, <2) 40% (237 to 143 per 10,000 discharges, 2-4) 51% (<14) 2 39·2% (111 to 67·6 per 10,000 discharges, <14) Prospective post-licensure studies Italy19 2+1 2001-2002 Germany20 3+1 2001-2002 (first dose) follow-up for 1 year postbooster Prospective cohort trial Interview of caregivers and pediatricians Physicians diagnosis 65% [47-78] (6-30 months) Non-randomised controlled Clinically diagnosed pneumonia 6·3% [-15·9 – 23·7] vaccine trial: Clinical pneumonia controlling for risk factors 24·8% [0·9-43·1] Children with underlying Clinical pneumonia in children with at least 1 risk factor: 38·4% [10·7-55·9] medical conditions Clinical pneumonia in children without risk factor: 10·9% [-20·2-33·9] preferentially included in PCV7 group IP = inpatient, OP = outpatient, ER = emergency department, Dx – diagnosis, VE = vaccine efficacy, RR= respiratory rate *non-bacteraemic pneumococcal pneumonia, ** results for 2001-2002 vs 2003-2004 comparison. No significant differences noted for 1998-199 vs 2003-204 comparisons. † pneumococcal and non-specific pneumonia, PPS = pneumococcal polysaccharide vaccine, ║bacterial pneumonia 3 References 1. De Wals P, Robin E, Fortin E, Thibeault R, Ouakki M, Douville-Fradet M. Pneumonia after implementation of the pneumococcal conjugate vaccine program in the province of Quebec, Canada. Pediatr. Infect. Dis. J. 2008 Nov;27(11):963– 968. 2. Jardine A, Menzies RI, McIntyre PB. Reduction in hospitalizations for pneumonia associated with the introduction of a pneumococcal conjugate vaccination schedule without a booster dose in Australia. Pediatr. Infect. Dis. J. 2010 Jul;29(7):607–612. 3. Grijalva CG, Poehling KA, Nuorti JP, Zhu Y, Martin SW, Edwards KM, et al. National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics. 2006 Sep;118(3):865–873. 4. Grijalva CG, Nuorti JP, Zhu Y, Griffin MR. Increasing incidence of empyema complicating childhood community-acquired pneumonia in the United States. Clin. Infect. Dis. 2010 Mar 15;50(6):805–813. 5. Zhou F, Kyaw MH, Shefer A, Winston CA, Nuorti JP. Health care utilization for pneumonia in young children after routine pneumococcal conjugate vaccine use in the United States. Arch Pediatr Adolesc Med. 2007 Dec;161(12):1162–1168. 6. Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007 Apr 7;369(9568):1179–1186. 7. Nelson JC, Jackson M, Yu O, Whitney CG, Bounds L, Bittner R, et al. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine. 2008 Sep 8;26(38):4947–4954. 8. Moore HC, Lehmann D, de Klerk N, Jacoby P, Richmond PC. Reduction in disparity for pneumonia hospitalisations between Australian indigenous and nonIndigenous children. J Epidemiol Community Health [Internet]. 2011 Jan 22 [cited 2011 Jun 20];Available from: http://www.ncbi.nlm.nih.gov/pubmed/21258115 9. O’Grady KF, Carlin JB, Chang AB, Torzillo PJ, Nolan TM, Ruben A, et al. Effectiveness of 7-valent pneumococcal conjugate vaccine against radiologically diagnosed pneumonia in indigenous infants in Australia. Bull. World Health Organ. 2010 Feb;88(2):139–146. 10. Ansaldi F, Sticchi L, Durando P, Carloni R, Oreste P, Vercelli M, et al. Decline in pneumonia and acute otitis media after the introduction of childhood pneumococcal vaccination in Liguria, Italy. J. Int. Med. Res. 2008 Dec;36(6):1255–1260. 11. Li S-TT, Tancredi DJ. Empyema hospitalizations increased in US children despite pneumococcal conjugate vaccine. Pediatrics. 2010 Jan;125(1):26–33. 12. Simonsen L, Taylor RJ, Young-Xu Y, Haber M, May L, Klugman KP. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. MBio. 2011;2(1):o00309–10. 13. Lee GE, Lorch SA, Sheffler-Collins S, Kronman MP, Shah SS. National hospitalization trends for pediatric pneumonia and associated complications. Pediatrics. 2010 Aug;126(2):204–213. 14. Pneumonia hospitalizations among young children before and after introduction of pneumococcal conjugate vaccine--United States, 1997-2006. MMWR Morb. Mortal. Wkly. Rep. 2009 Jan 16;58(1):1–4. 15. Kronman MP, Hersh AL, Feng R, Huang Y-S, Lee GE, Shah SS. Ambulatory visit rates and antibiotic prescribing for children with pneumonia, 1994-2007. Pediatrics. 2011 Mar;127(3):411–418. 16. Koshy E, Murray J, Bottle A, Sharland M, Saxena S. Impact of the seven-valent pneumococcal conjugate vaccination (PCV7) programme on childhood hospital admissions for bacterial pneumonia and empyema in England: national time-trends study, 1997-2008. Thorax. 2010 Sep;65(9):770–774. 17. Patrzałek M, Albrecht P, Sobczynski M. Significant decline in pneumonia admission rate after the introduction of routine 2+1 dose schedule heptavalent pneumococcal conjugate vaccine (PCV7) in children under 5 years of age in Kielce, Poland. Eur. J. Clin. Microbiol. Infect. Dis. 2010 Jul;29(7):787–792. 18. Pírez MC, Algorta G, Cedrés A, Sobrero H, Varela A, Giachetto G, et al. Impact of Universal Pneumococcal Vaccination on Hospitalizations for Pneumonia and Meningitis in Children in Montevideo, Uruguay. Pediatr Infect Dis J. 2011 Mar 14;30(8):669–74. 19. Esposito S, Lizioli A, Lastrico A, Begliatti E, Rognoni A, Tagliabue C, et al. Impact on respiratory tract infections of heptavalent pneumococcal conjugate vaccine administered at 3, 5 and 11 months of age. Respir. Res. 2007;8:12. 20. Adam D, Fehnle K. Safety and effectiveness against respiratory tract infections for pneumococcal conjugate vaccine co-administered with routine vaccine combinations. Vaccine. 2008 Nov 5;26(47):5944–5951.