More Info - Vivione Biosciences

empiriSTAT – Pneumonia™
Overview
empiriSTAT™ is a rapid antimicrobial susceptibility assay meant to optimize upfront empiric therapy in pneumonia patients by use of the RAPID-B platform.
Current Methods
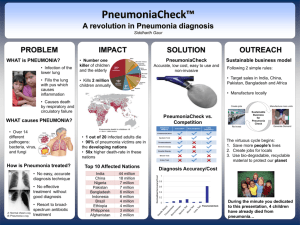
While the diagnosis of pneumonia is accomplished through clinical symptoms and chest radiograph, for hospital-associated pneumonia blood culture is considered a standard method for determining the causative agent associated with the infection.
A standard approach to treating pneumonia while awaiting blood culture results is to provide empiric, broad-spectrum antibiotics while the microbiology is performed.
This “blind” approach to therapy is used to mitigate risk in clinical scenarios, with the intention that broad-spectrum therapy will be discontinued and focused therapy will be utilized once bacteria is isolated and antibiotic susceptibility testing can be accomplished. But this approach offers risk that the chosen therapy is ineffective and possibly contributing to the growth of resistant bacteria.
empiriSTAT™
The empiriSTAT™ test aims to provide clinicians rapid quantitative results regarding the effect of standard empiric therapies defined in pneumonia guidelines, such as the Infectious Disease Society of America Guidelines for Nosocomial Pneumonia.
The test will provide quantitative reductions in bacterial growth due to standard empiric antimicrobial therapies relative to a control sample with no antibiotics in the growth media.
Therapies Assessed by empiriSTAT
Class
β-lactam/β-lactamase Inhibitor
Drug
Ampicillin/Sulbactam
β-lactam/ β-lactamase Inhibitor
Cephalosporin
Fluoroquinolone
Fluoroquinolone
Fluoroquinolone
Carbapenem
Glycopeptide
RAPID-B Platform
Piperacillin/Tazobactam
Ceftriaxone
Levofloxacin
Ciprofloxacin
Moxifloxacin
Ertapenem
Vancomycin
RAPID-B represents a fully automated and informatics integrated platform for microbiology. The system utilizes sample cassettes for sample loading and, where appropriate, antibiotics for susceptibility results.
Market Landscape
The market opportunity for a rapid technology aimed at bacterial detection in blood is substantial. The company feels that RAPID-B’s analytical sensitivity will translate into both general bacterial presence results AND drug susceptibility results in under
6 hours, which in todays technology landscape is a game changing approach to the detection of bacteria in blood samples. Currently this aspect of microbiology is performed using CO2 sensing technologies represented by BD, Biomerieux, and
Thermo Scientific/ TREK.
Current workload involves primary sample processing,, plating upon “beep” positive samples, and colony picking after incubation. Estimates on lab expense are in the range of $40-60/sample, which includes disposables and labor expenses.
At this point in time the RAPID-B technology is not focusing on speciation. Current technologies involved in this aspect of microbial identification include MALDI-TOF
(Biomerieux, Brucker), PCR enabled MALDI-TOF (Abbott/Iridica), PCR (Cepheid,
Roche, BD), and traditional Gram staining with chemical analysis.
Market analysis has determined that antibiotic susceptibility testing (AST) is the primary piece of useful information coveted by acute care clinicians. The investment value of rapid AST can be seen in the market cap of Accelerate Diagnostics, valued at over $1B prior to FDA approval. The company is convinced that the RAPID-B AST approach is more accurate, faster, and more comprehensive than Accelerate Dx approach.