FLAME TEST LAB
advertisement

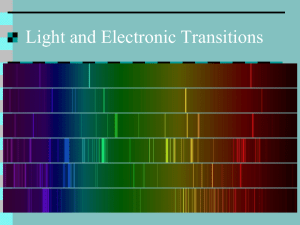
NAME: PER: FLAME TEST LAB – Atomic Emission and Electron Energy Levels Introduction Just as a fingerprint is unique to each person, the color of light emitted by an element heated in a flame is also unique to each element. In this experiment, the characteristic color of light emitted by sodium, lithium, copper, calcium, strontium, potassium and barium ions will be analyzed. In addition, an unknown ion will be analyzed along with a control group containing no ions. The color seen in each test is the sum of all of the wavelengths emitted by each element. Figure 1: An atom emits light when its electrons make the transition from excited energy states to lower energy states. Concepts Atomic emission Excited vs. ground states Wavelength, frequency and energy of light Flame tests Background When a substance is heated in a flame, the atoms absorb energy from the flame. This absorbed energy causes the electrons to be promoted to excited energy levels. From these excited energy levels, the electrons have the natural tendency to quickly drop back down to lower energy states. When an electron makes the transition from a higher energy level to a lower energy level, a particle of light, called a photon is emitted (see Figure 1). Both the absorption and emission of energy are quantized – only certain energy levels are allowed. An electron may drop all the way back down to the ground state in a single step, emitting a photon in the process. Alternatively, an electron may drop back down to the ground state in a series of smaller steps, emitting a photon with each step. In either case, the energy of each emitted photon is equal to the difference in energy between the excited state and the state to which the electron relaxes. The energy of the emitted photon determines the color of light observed in the flame. The flame color may be described in terms of its wavelength, frequency and energy using the following light equations: 1. 2. 3. c = λν E = hν E = h c λ USEFUL LIGHT CONSTANTS c = 3.0 x 108 m/s h = 6.626 x 10 -34 J·s The color of light observed when a substance is heated in a flame varies from one substance to another, because each element’s electron’s are organized differently, making their transitions from excited energy states to lower energy states unique. Therefore, the difference in energy between energy levels, the exact energy of the emitted photon, and the corresponding wavelength and color are unique to each substance. As a result, the colors observed when a substance is heated in a flame may be used as a means of identification. Experiment Overview The purpose of this experiment is to observe the characteristic flame test colors of different metal compounds and to use this information to identify an unknown cation. Prelab questions 1. Fill in the blanks: When an atom absorbs energy, the electrons move from their state to a(n) state. When an atom emits energy, the electrons move from a(n) give off state to their and . 2. Is a flame test a qualitative or quantitative test for the identity of the unknown. Materials SAFETY PRECAUTIONS! Avoid ingestions and contact with eyes and for all solutions. Fully extinguish wooden splints by immersing them in a beaker of water. Do not dispose of them in a trash can to avoid accidental fires. Wear safety goggles at all times. Wash hands with soap and wter when finished with experiment. Procedure 1. Light the Bunsen burner 2. Take the soaked end of one of the wooden splints resting in the flask of a metal chloride solution and place it in the flame. Observe the color of the flame. Allow the splint to burn until the color fades. Try not to allow any solids to fall into the burner itself. 3. Place the wooden splint in the waste beaker filled with water. 4. Record your observations for the flame color in the data table provided 5. Repeat steps 1-4 for all solutions except the unknown ion solution. 6. Perform a flame test on the unknown ion solution, record its characteristic color and determine its probable identity based upon earlier observations. Data Table Metal Ion Color of Flame sodium lithium copper calcium potassium strontium barium control unknown Probable identity of unknown ion = λ (nm) λ (m) E (J) Postlab Questions 1. Complete the data table column labeled “Wavelength (nm) “ using the EM spectrum diagram from pg 306 in your textbook 2. Convert each wavelength in the data table from nm to m. Show one (and only one) example calculation below. 3. Calculate the energy of the light emitted for each sample in the data table using light equation #3. Show one (and only one) example calculation below. 4. What evidence is there from your results that the characteristic color observed for each compound is due to the cation in the solution, not the anion? Describe an additional test that could be used to confirm that the color is due to the metal ion. 5. The alkali metals cesium (Cs) and rubidium (Rb) were discovered based on their characteristic bright flame colors. Cesium is named after the sky and rubidium after the gem. What colors due you think each of these metals might give off in a flame test?