Chemical oxygen demand (COD)
advertisement
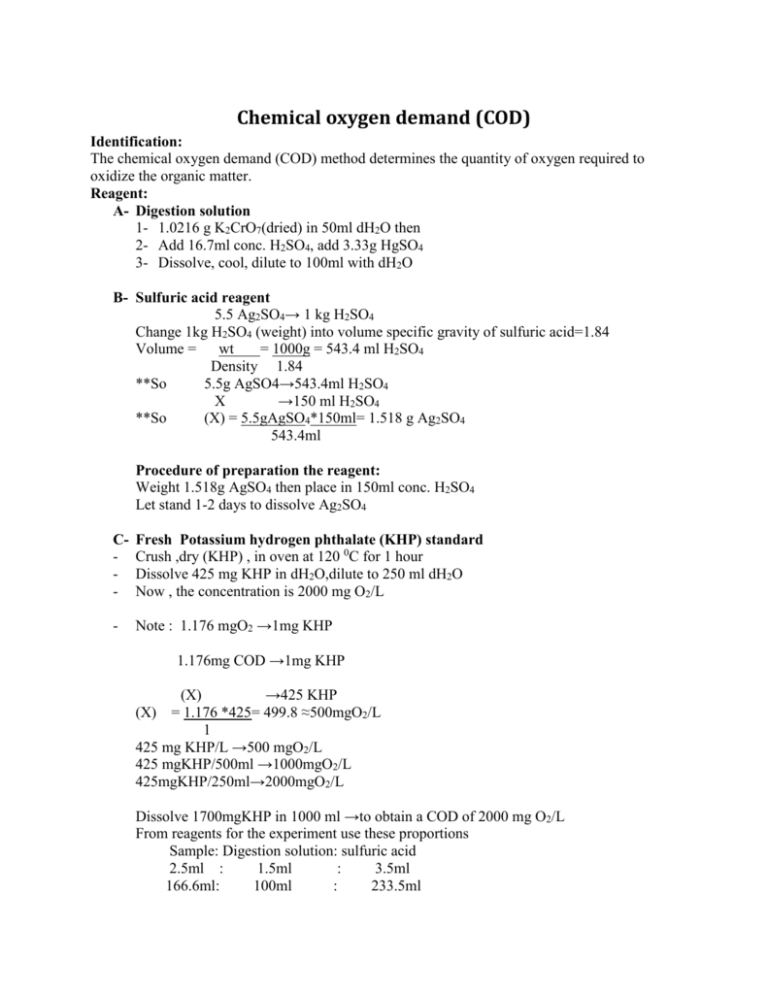
Chemical oxygen demand (COD) Identification: The chemical oxygen demand (COD) method determines the quantity of oxygen required to oxidize the organic matter. Reagent: A- Digestion solution 1- 1.0216 g K2CrO7(dried) in 50ml dH2O then 2- Add 16.7ml conc. H2SO4, add 3.33g HgSO4 3- Dissolve, cool, dilute to 100ml with dH2O B- Sulfuric acid reagent 5.5 Ag2SO4→ 1 kg H2SO4 Change 1kg H2SO4 (weight) into volume specific gravity of sulfuric acid=1.84 Volume = wt = 1000g = 543.4 ml H2SO4 Density 1.84 **So 5.5g AgSO4→543.4ml H2SO4 X →150 ml H2SO4 **So (X) = 5.5gAgSO4*150ml= 1.518 g Ag2SO4 543.4ml Procedure of preparation the reagent: Weight 1.518g AgSO4 then place in 150ml conc. H2SO4 Let stand 1-2 days to dissolve Ag2SO4 C- Fresh Potassium hydrogen phthalate (KHP) standard Crush ,dry (KHP) , in oven at 120 0C for 1 hour Dissolve 425 mg KHP in dH2O,dilute to 250 ml dH2O Now , the concentration is 2000 mg O2/L - Note : 1.176 mgO2 →1mg KHP 1.176mg COD →1mg KHP (X) →425 KHP = 1.176 *425= 499.8 ≈500mgO2/L 1 425 mg KHP/L →500 mgO2/L 425 mgKHP/500ml →1000mgO2/L 425mgKHP/250ml→2000mgO2/L (X) Dissolve 1700mgKHP in 1000 ml →to obtain a COD of 2000 mg O2/L From reagents for the experiment use these proportions Sample: Digestion solution: sulfuric acid 2.5ml : 1.5ml : 3.5ml 166.6ml: 100ml : 233.5ml D- Working solution standard mls of working std. dH2O 1000mg O2 0 10 0.2 9.8 0.5 9.5 1.0 9.0 2.0 8.0 3.0 7.0 5.0 5.0 7.5 2.5 9.0 1 Total volume Final conc. mgO2/L 10 10 10 10 10 10 10 10 10 0 20 50 100 200 300 500 750 900 Dilute 50 ml of stock standard up to 100ml to obtain a COD of 1000 mgO2/L Or: you can make a serial dilution as follows directly from working solution (1000mgO2/L) 1000mg O2/L(50ml) = 500 mg O2/L 100ml Sample preparation Shake the sample well (refers to table) Using16*100 mm tubes with Teflon screw, caps, Pyrex (150 0C) Digestion vessel Sample (ml) 2.5 Digestion solution (ml) 1.5 Sulfuric acid reagent (ml) 3.5 Total final volume (ml) 7.5 Culture tubes 16*100mm 20*150 25*150 Standard 10ml ampoules 5.0 10.0 2.5 3.0 6.0 1.5 7.0 14.0 3.5 15.0 30.0 7.5 - 2.5 ml sample (water or sewage water) 1.5ml digestion solution (K2Cr2O7+ HgSO4) 3.5ml H2SO4 +Ag2SO4 reagent **close the tube tightly (Check for Teflon) Mix gently by tilting and mixing, check for leaks - Place tubes in heating block at 150 0C - Allow to reflux for 2 hours - Remove from the heating block place in a rock, cool to room temperature. - * read at λ=600nm ,adjust zero absorbance for reagent blank Samples are done in duplicate (or triplication) For high values > than 900 mg O2 dilute 1+4(1:5) Or (1:10)1+9 with dH2O ** Plot absorbance vs. concentration








