CE and IC
advertisement

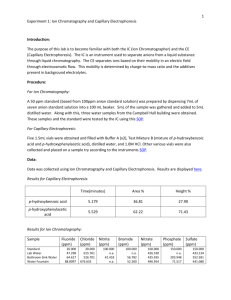
Naomi Bryner CE and IC Lab Week of 3/25/13 Introduction: Ion chromatography (IC) is a separation process for aqueous solutions based on sample charge. Ionic species of a sample interact differently within the stationary phase as the sample is forced through a column. These differences between the sample and stationary phase’s level of interaction are due to the species’ various charges and sizes. The concentration of analyte is detected by UV/Vis absorbance or conductivity, and the retention time of the each ion is used for identification purposes. Capillary electrophoresis (CE) operates based on the mass-to-charge ratio of each species within a sample. This ratio coupled with the electrolyte which fills the capillary and the electric field applied to the capillary allows for separation of species. Migration time of each species appear as peaks on the produced electropherogram. Electrolyte flow can be replaced with a buffered gel matrix, allowing for separation of tiny molecules such as DNA strands or proteins. CE improves significantly upon the speed and resolution of traditional slab-style gel electrophoresis. Purpose: This lab is designed to become familiar with and run an experiment on the CE and IC. The IC will be used to analyze a seven-anion sample as a standard and various tap water samples as unknowns. The CE will be used to separate unknown mixtures of the known acids, p-hydroxybenzoic and phydroxyphenylacetic acid. Procedure: Ion Chromatography: 1. Turn on helium in gas room and behind instrument 2. Check helium tank pressure (80 psi) and pressure on instrument (9 psi) 3. Ensure that regenerant and eluent bottles are more than half full a. If less than half full, they must be refilled 4. Equilibrate the instrument a. After 20-30 min, test flow rate (1 mL/min) 5. Set up the software 6. Run the Sequence a. Inject sample when prompted to do so Run four water samples salt water or flavored water can deteriorate the instrument b. Each run should take 16 min 7. Analyze Peaks 8. Shut Down a. Select Stop in Batch program b. Leave Chromeleon running c. Be sure to turn off the helium knob behind the instrument Capillary Electrophoresis: Solution Prep: 1. Capillary Performance Test Mixture B (mixture of p-hydroxybenzoic acid and phydroxyphenylacetic acid) 2. Capillary Performance Run Buffer A (pH 8.35) 3. Capillary Electrophoresis System Solution A (0.1 M NaOH) 4. Distilled water 5. 1.0 M HCl Solution 6. All chemicals should be stored in the chemical refrigerator or under the instrument Instrumentation: 1. Turn on the instrument, allow for 30 minute warm-up 2. Read Dr. Hu’s SOP while the instrument is warming up a. Prepare and label chemicals b. Load buffer and sample vials according to Dr. Hu’s SOP 3. Build Method using Dr. Hu’s SOP a. Time Program: i. Rinse 1.0 min (20 psi, forward pressure) from distilled water to Waste ii. Rinse 2.0 min (20 psi, forward pressure) from 0.1 M NaOH to Waste iii. Rinse 1.0 min (20 psi, forward pressure) from distilled water to Waste iv. Rinse 2.0 min (20 psi, forward pressure) from Buffer A to Waste v. Inject (0.5 psi pressure, 10 seconds) from sample vial (Test Mixture B) to Waste vi. Separate at 0.0 min (Duration: 7 min, Voltage: 25 KV, Ramp: 0.17 min) from Run Buffer A (BI) to Run Buffer A (BO) vii. Autozero at time 1.50 min viii. Stop data at 7.0 min ix. Rinse 1.0 min at time 8.0 min (20 psi, forward pressure) from distilled water to Waste 4. Save and Run Method 5. Save and print electropherogram with report 6. If you are the last group, exit the program and turn off the instrument a. Remove all vials, except the buffer vials 7. Load the trays before turning off the instrument Data: Capillary Electrophoresis Area % Report can be found on page 33 Prominent Peaks (min) Area Area % 5.308 110826 34.05 5.700 169710 52.15 Ion Chromatography IC data can be found on page 33 1. Seven Anion Standard Retention Time (min) Peak Name Amount (ppm) 4.22 Fluoride 1 4.76 Chloride 1 6.59 Nitrite 1 7.61 Bromide 1 9.41 Nitrate 1 10.40 Phosphate 1 13.59 Sulfate 1 2. Water Fountain Retention Time (min) Peak Name Amount (ppm) 4.77 Chloride 0.779 6.60 Nitrite 0.925 7.62 Bromide 4.139 9.40 Nitrate 4.432 10.38 Phosphate 1.083 13.59 Sulfate 4.937 3. Lab Sink Retention Time (min) Peak Name Amount (ppm) 4.76 Chloride 0.201 6.59 Nitrite 0.704 7.60 Bromide 0.800 9.37 Nitrate 1.285 10.35 Phosphate 0.719 13.57 Sulfate 1.069 4. Kenmore Refrigerator Filter Retention Time (min) Peak Name Amount (ppm) 4.78 Chloride 0.692 6.60 Nitrite 0.635 7.61 Bromide 0.551 9.40 Nitrate 0.762 10.39 Phosphate 0.590 13.62 Sulfate 0.745 5. Bathroom Sink Retention Time (min) Peak Name Amount (ppm) 6.62 Nitrite 0.802 10.41 Phosphate 0.758 6. Brita Filter Retention Time (min) Peak Name Amount (ppm) 6.59 Nitrite 1.164 10.36 Phosphate 0.749 Conclusions: On the CE, test mixture B was examined, which was known to be a mixture of p-hydroxybenzoic acid and p-hydroxyphenylacetic acid. The first peak appeared at 5.308 min, and was the lightest molecule, p-hydroxybenzoic acid. The second peak appeared at 5.700 min, and was the heavier molecule, p-hydroxyphenylacetic acid. Percent area is equal to percent composition, so it is known that there is 34.05% p-hydroxybenzoic acid and 52.15% p-hydroxyphenylacetic acid in text mixture B. On the IC, a seven anion standard was used to calibrate the instrument. Unknown water samples came from the water fountain in Campbell Hall, the sink in the Instrumental Lab, the spout of a Kenmore refrigerator (filtered), the water from the bathroom, and a Brita water bottle filter. It was seen that the Brita filter and bathroom water had the least contaminants.