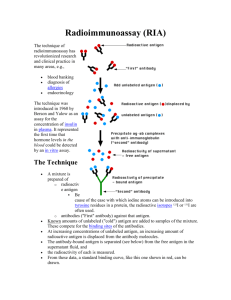
Supplementary Data

Supplementary Table 1 Post mortem delay and fixation duration of all cases
Supplementary Table 2 Antibodies and protocols for immunohistochemical studies. Apart from those antibodies listed, other anti-P-glycoprotein antibodies (MRKm6, MM12, MM14), anti-BCRP (BXP34), anti-MRP1 (QCRL), anti-MRP2 (M2I4, M2III6), anti-claudin (1, 8) and anti-connexin (32, 36) were also tested but they were not used in the present study because they failed to give specific immunopositive labelling in formalin-fixed, surgical and/or post-mortem positive controls despite using various enzyme, citrate-buffered microwave and pressure cooker antigen retrieval methods and differing incubation time and temperatures.
Automated immunohistochemistry was performed using a Bond Max Automated
Immunostainer and reagents (Leica, UK). Sections were first processed in dewaxing solution and 100% alcohol, and immersed in 3% hydrogen peroxide solution for five minutes. Antigen retrieval and primary antibodies incubation protocols were applied as above. Post-primary polymer solutions were applied for eight minutes before chromogen activation was achieved using diaminobenzidine. In manual immunohistochemistry, paraffin-embedded sections were immersed in xylene and graded concentrations of alcohol before incubating in 3% hydrogen peroxide solution diluted in de-ionised water for fifteen minutes. Antigen retrieval and primary antibodies incubation protocols were applied as above. On the following day, Dako
Envision horseradish peroxide solution was applied for thirty minutes. Dako Envision diaminobenzidine and substrate buffer were then added for five minutes. All sections were washed in PBS-T between each step. Sections where primary antibodies were omitted were included in each experiment as negative control. Sections were counterstained in ready-to-use hematoxylin (VWR, USA) for a minute and were differentiated in distilled water, ascending concentrations of alcohols and xylene before coverslipping (TissueTek).
&
Prior to study, C494 immunopositive labelling was compared to the immunolabelling of two other well-studied anti-P-glycoprotein clones, JSB and C219, to ensure C494 specifically and faithfully detect P-glycoprotein expression in surgical and post-mortem human brain tissue.
*Labelling was similar with or without antigen retrieval pretreatment. **Antigen retrieval pretreatment introduced non-specific staining and so pretreatment protocols were not applied.
^The expression of Cx43 is believed to be associated with the spread of inflammation and/or seizures after acute brain injury and epilepsy (Chew et al ., 2010, Collignon et al ., 2006,
Cronin et al ., 2008, Fonseca et al ., 2002) so we examined the immunolabelling of anti-Cx43 in the present study. BCRP= breast cancer resistance protein, Cx43= connexin 43, ER1 or 2= antigen retrieval reagent 1 or 2, GFAP=glial fibrillary acidic protein, MRP1= multidrug resistance-related protein 1, P-glycoprotein= P-glycoprotein, VUmc = VU Medical Centre,
Amsterdam.
References:
Chew SS, Johnson CS, Green CR, et al . Role of connexin 43 in central nervous system injury. Exp Neurol 2010; 225: 250-261
Collignon F, Wetjun NM, Cohen-Gadol AA, et al . Altered expression of connexin subtyes in mesial temporal lobe epilepsy in humans. J Neurosurg 2006; 105: 77-87
Cronin M, Anderson PN, Cook JE et al , Blocking connexin43 expression reduces inflammation and improves functional recovery after spinal cord injury. Molecular and
Cellular Neuroscience 2008; 39: 152-160
Fonseca CG, Green CR, Nicholson LF, et al . Upregulation in astrocytic connexin43 gap junction levels may exacerbate generalized seizures in mesial temporal lobe epilepsy. Brain
Res 2002; 929: 105-116
Supplementary Fig S1 Sampling for quantitative analysis. Using the imaging software,
ImagePro Plus (Mediacybernetics, UK), we found that the random systematic sampling scheme that sampled 10% of the total area of interest gave similar results as if sampling was performed at 92% (A, EP266) or 95% (B, EP286). Therefore, sampling at 10% was used in the final trial. (C) We did not find the percentage area of anti-P-glycoprotein or anti-BCRP immunolabelling to be different in different sections cut from the same block, so only one section from each region of each case was immunolabelled and quantified.
Supplementary Table 3 Previous studies that have shown certain anti-epileptic drugs (AED) are inducers and/or substrates of P-glycoprotein; however, this is still an ongoing debate
(Löscher et al ., 2011; Zhang et al ., 2011). Abbreviations: CBZ= carbamazepine; LEV= levetiracetam; LTG= lamotrigine; OXC= oxcarbazepine; PB= Phenobarbital; PHT= phenytoin; TPM= topiramate; VPA= valproate