7a ELISA Test
advertisement

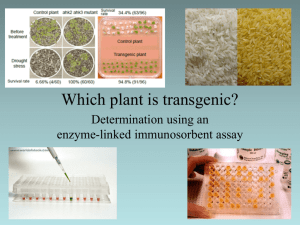
ELISA TEST Enzyme-linked immunosorbent assay (ELISA) is a biochemical technique used mainly in immunology to detect the presence of an antigen in a sample. An unknown amount of antigen is affixed to a surface, and then a primary antibody is applied over the surface so that it can bind to the antigen. This antibody is linked to an enzyme, and, in the final step, a substance containing the enzyme's substrate is added. The subsequent reaction produces a detectable signal, most commonly a color change in the substrate. Enzyme: horseradish peroxidase Secondary antibody Either antigen or primary antibody ELISA tests could also use an antibody instead of the antigen. In this case, there will be two sets of antibodies, so we call them primary and secondary antibodies. The primary antibodies will be attached to the plastic plate, and then the secondary antibodies will attach to the primary antibodies. The secondary antibodies will then be conjugated to the enzyme, horseradish peroxidase, which will create a color change when a substrate is added. An ELISA test can tell us whether or not particular antigens or antibodies are present in the sample (qualitative). However, we cannot measure how many antigens or antibodies are present (quantitative) unless we perform a serial dilution. For this exercise, you will receive a fluid sample that you pretend is from your body. It is labeled with a number; write that down. One of these samples is positive for an antigen (we are pretending it is positive for HIV). Then you will go around the class (between decks too!) and pretend to transfer body fluids. You put your fluid in someone else’s tube, mix it, and take half of it back. After the whole class has donated once, then go around and donate a second time. Keep track of who you donated to, and who you received donations from. You can donate only twice but you can be the recipient as many times as you want. After performing the ELISA test, if your sample is positive, track down where you might have gotten it from. Try to figure out who had the positive sample in the beginning. Procedure The 96 well plates have letters down the left side and numbers across the top. ELISA uses positive and negative controls. The first three wells on the top row are used for positive controls, the next three for negative controls. The patient’s fluid samples are done in triplicate, so each student will take up three wells. Decide with the other people in your deck as to who will use which of the leftover wells in the plate; write your well numbers down on a table. ELISA’s are run in triplicate for several reasons: to circumvent the possible false negatives, to circumvent the possible false positives, and to increase statistical significance of the reactions. Once everyone in your deck has added their fluid to their assigned wells, let them incubate for 5 minutes and wash it with a buffer that will wash out any unbound antigen. Then put in a primary antibody (since we are pretending the antigen is HIV, the primary antibody would have to be an anti-HIV antibody). Let it incubate for 5 minutes to allow it to bind to the antigen if the antigen is present. Wash again with wash buffer, which will wash away any unbound antigen. Then add a secondary antibody. These are anti-human antibodies; antibodies against human antibodies. These secondary antibodies also have a horseradish peroxidase (HRP) enzyme attached to them. Allow the tray to incubate another 5 minutes, then wash with the buffer. Now add the substrate, which will bind to the HRP if HRP is present. When HRP comes in contact with the substrate, the color changes to blue. If the blue color appears, it means that the substrate found HRP to bind to. If HRP is present, the secondary antibodies must be present. If the secondary antibodies are present, that means the primary antibodies are present. If the primary antibodies are present, that means the antigen is present, so a color change is positive for the antigen (which we are pretending is HIV).