File
advertisement
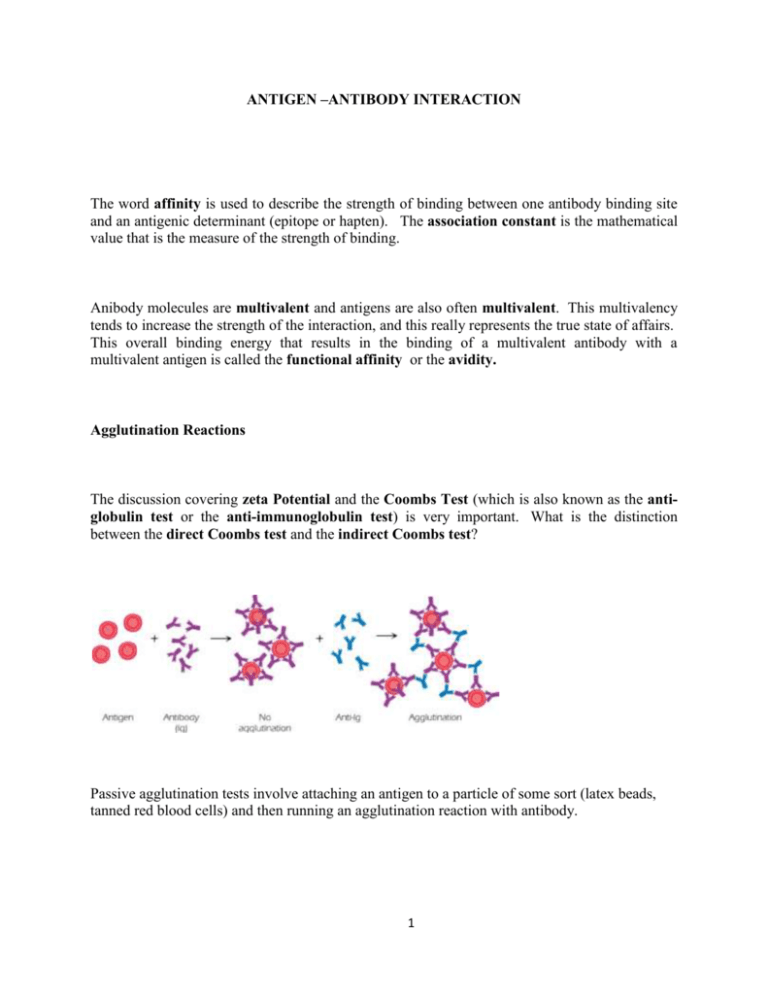
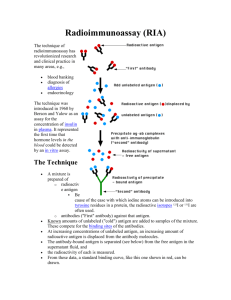
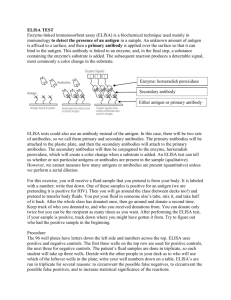
ANTIGEN –ANTIBODY INTERACTION The word affinity is used to describe the strength of binding between one antibody binding site and an antigenic determinant (epitope or hapten). The association constant is the mathematical value that is the measure of the strength of binding. Anibody molecules are multivalent and antigens are also often multivalent. This multivalency tends to increase the strength of the interaction, and this really represents the true state of affairs. This overall binding energy that results in the binding of a multivalent antibody with a multivalent antigen is called the functional affinity or the avidity. Agglutination Reactions The discussion covering zeta Potential and the Coombs Test (which is also known as the antiglobulin test or the anti-immunoglobulin test) is very important. What is the distinction between the direct Coombs test and the indirect Coombs test? Passive agglutination tests involve attaching an antigen to a particle of some sort (latex beads, tanned red blood cells) and then running an agglutination reaction with antibody. 1 Precipitation Reactions This describes the reaction between soluble antibody and soluble antigen in which an insoluble product results. (Remember that precipitation is a secondary phenomenon. Ag-Ab reactions may occur and form soluble immune complexes even without the production of a visible precipitate!) Precipitation reactions can be done in a variety of ways: In test tubes In agarose gels: As double diffusion As single diffusion After electrophoresis Immunoassays Solid phase immunoassays; ELISA – Enzyme linked immunosorbant assay 1. You need reagent antibodies or reagent binding proteins that have been “tagged” with an enzyme label. This means that the enzyme has been covalently coupled to the protein reagent. a. Typical enzymes include: Horseradish peroxidase or Alkaline phosphatase 2 b. Reagent proteins include antibodies such as goat-anti-human-IgG. Or, bacterial proteins that bind to antibodies such as Staphylococcal protein A or Streptococcal protein G. Biotin and Avidin can also be used ---- avidin has several high-affinity binding sites for biotin thus it can be used to bridge molecules that have been “tagged” with biotin. 2. You need some kind of “solid phase” to which proteins can stick. This would usually be some kind of plastic microtiter plate. Thus, as is the example in Fig 5.11, antigen can be used to “naturally” coat the wells of a microtiter plate. After you do this you would have to “block” the plate with some kind of irrelevant protein (or detergent). We usually use a milk solution for this that is called BLOTTO. 3. One then reacts the antigen-coated plate with appropriate enzyme-tagged antibody. (You can actually sandwich several different reagents at this point – as will be discussed in class.) Afterwards the appropriate substrate is added and a color-change will indicate a “positive” test. 3 Western Blot In these assays, antigens are first electrophoresed and then blotted onto a piece of filter-paper. Thus the filter paper is the solid phase to which the antigen is bound. The filter is blocked with BLOTTO and then reacted with appropriate “tagged” antibodies and then substrate. As above, a color reaction indicates a “positive” test. Immunofluorescence 4 In these assays, antibodies or other reagent proteins are “tagged” or labeled with fluorescent dyes. These fluorescent reagents can then be used to stain samples mounted onto microscope slides and the slides can be examined using a fluorescent microscope. Typical fluorescent labels include FITC, TRITC, PE and many others. Cells in suspension can also be stained fluorescently and then analyzed by fluorescenceactivated cell sorting or FACS analyis. 5 Reagent Antibodies For many of the serological tests described above, it is essential to have antibodies of defined specificity which can be used as reagents in the tests. Traditionally such antibodies were made as polyclonal antibodies. To make such antibodies, very pure antigen was injected into subject animals and then after an appropriate amount of time (often after several “booster shots”) the antibody containing serum was harvested from the animal by bleeding. Such polyclonal antisera are very valuable, but they do have some limitations. Particularly, such antisera contain populations of antibodies that react to all the epitopes of the antigen prep – including any impurities that might have contaminated the antigen. Of course, polyclonal antisera also contain multiple isotypes of antibody too. 6 To get around this problem, one can immunize an animal and then remove and culture and clone its B-cells. In this technique, one then cultures single clones of B-cells, each clone producing only one specificity of antibody, reactive with only one epitope of the immunizing antigen. This procedure involves a number of sophisticated tricks that we will list here and discuss in more detail in class. 1. Normal B-cells will not survive very long in culture so one must immortalize them by giving them genes from cancerous B-cells. Cancerous plasma cells (antibody secreting B-cells) are called myeloma cells. B-cells from immunized animals are mixed with myeloma cells in the presence of a mild detergent (polyethylene glycol or PEG). This causes cells to fuse forming hybridomas or hybrids between normal and myeloma cells. 2. A selection system is used that preferentially allows the hybridoma cells to grow while suppressing the growth of any non-hybridized myeloma cells. 3. Hybridoma cells are fragile, so feeder cells of macrophages are used to produce growth promoting cytokines. 4. Appropriate immunoassays (usually ELISA’s) have that allow you detect any antibodies being produced by the hybridoma clones. 7 8