Old Exam
CHE 230
Exam #3 Spring 2011
Name _______________________ **Record your name here AND on the IFAT sheet**
A. Multiple Choice. For each of the following multiple choice questions, use the IFAT sheets to make your selection. When you get to the correct answer (indicated with a star), record your score for that question:
3 pts if right on first try , 2 pts for second try , 1 pt for third try , 0 pts for fourth try
Once you have the correct answer, also write it in on the paper exam.
**If for some reason, you think there is not a right answer (or the wrong answer is indicated to be correct), please write on the exam what you think the right answer is and show your work.**
1. _______ What is the approximate energy of red light?
A. 4*10 -19 J
B. 3*10 -19 J
C. 4*10 -28 J
D. 3*10 -28 J
2. _______ According to the literature, chlorophyll a (893.51 g/mol) has a molar extinction coefficient of 111,700
M*cm -1 at 417.8 nm. What approximate concentration of chlorophyll a should you prepare to observe an absorbance of 0.1 in a standard cuvette at 417.8 nm?
A. 1*10 -9 M
B. 9*10 -7 M
C. 2*10 -9 M
D. There is not enough information to calculate the approximate concentration.
3. _______ Which of the following best characterizes a reverse phase liquid chromatography system?
A. Nonpolar stationary phase and polar mobile phase
B. Nonpolar stationary phase and nonpolar mobile phase
C. Polar stationary phase and nonpolar mobile phase
D. Polar stationary phase and polar mobile phase
4. _______ Benzoic acid is a weak organic acid with a pKa of 4.2. It can be analyzed using HPLC separation with a 150 mm C18 column and an isocratic mobile phase of acetate buffer at pH of 3.8. At a flow rate of 1.0 mL/min, the retention time for benzoic acid is 3.7 min. If the mobile phase is changed to a phosphate buffer at a pH of 7.0 (but all the other conditions are unchanged), how will the retention time for benzoic acid be affected?
A. The retention time will be more difficult to determine because the peak will broaden.
B. The retention time will increase.
C. The retention time will stay the same.
D. The retention time will decrease.
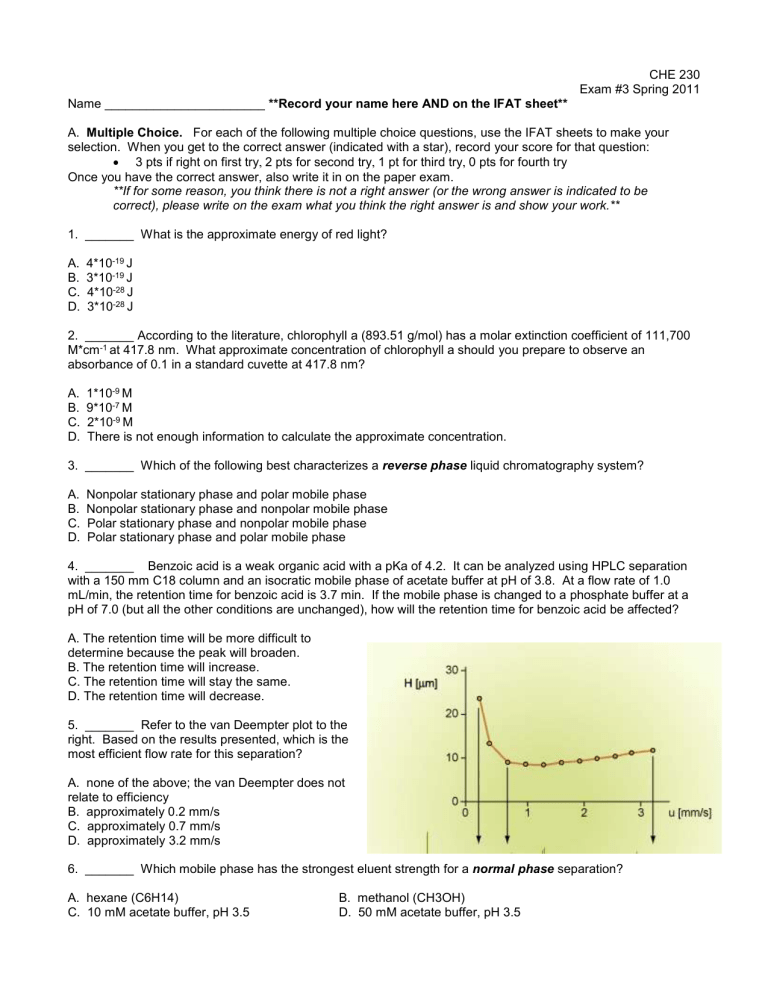
5. _______ Refer to the van Deempter plot to the right. Based on the results presented, which is the most efficient flow rate for this separation?
A. none of the above; the van Deempter does not relate to efficiency
B. approximately 0.2 mm/s
C. approximately 0.7 mm/s
D. approximately 3.2 mm/s
6. _______ Which mobile phase has the strongest eluent strength for a normal phase separation?
A. hexane (C6H14)
C. 10 mM acetate buffer, pH 3.5
B. methanol (CH3OH)
D. 50 mM acetate buffer, pH 3.5
Name
Structure
Phenol Naphthalene 1-naphthol
Density (g/mL)
Melting point (˚C)
Boiling point (˚C)
Solubility in water
Formula
Molecular weight (g/mol)
C6H5OH
94.11
1.06
43
182
1 g per 15 mL
C10H8
128.17
1.14
80
218
30 mg/L
C10H8O
144.17
1.10
95
278
Slightly soluble in water
7. _______ Refer to the three compounds listed above. Rank these compounds in order of increasing retention time in a standard gas chromatography system.
A. Phenol < 1-naphthol < Naphthalene
B. Naphthalene < Phenol < 1-naphthol
C. Phenol < Naphthalene < 1-naphthol
D. 1-naphthol < Phenol < Naphthalene
8. _______ Refer to the three compounds listed above. Rank these compounds in order of increasing retention time in a reverse phase liquid chromatography system.
A. Phenol < Naphthalene < 1-naphthol
B. Naphthalene < Phenol < 1-naphthol
C. 1-naphthol < Phenol < Naphthalene
D. Phenol < 1-naphthol < Naphthalene
9. _______ Methyl amine is a weak organic base with a pKa of 10.64. Ion exchange chromatography could be used to purify this weak base. Which of the following statements best describes the ion exchange process for methyl amine?
A. Methyl amine will adsorb to a cation exchange resin at a pH of 3 and elute at a pH of 7.
B. Methyl amine will adsorb to a cation exchange resin at a pH of 7 and elute at a pH of 3.
C. Methyl amine will adsorb to a cation exchange resin at a pH of 7 and elute at a pH of 12.
D. Methyl amine will adsorb to a cation exchange resin at a pH of 12 and elute at a pH of 7.
10. _______ The “golden triangle of chromatography” illustrates that:
A. efficiency, resolution, and analysis time should all be optimized for a given separation.
B. efficiency, flow rate, and analysis time should all be optimized for a given separation.
C. efficiency and flow rate can be optimized at the expense of the analysis time.
D. efficiency and resolution can be optimized at the expense of the analysis time.
11. _______ In the Jablonski diagram of energy transitions, non-radiative decay refers to:
A. the forbidden spin flip that occurs prior to the process of phosphorescence.
B. the emission of light in the UV region (where the emission can’t be seen).
C. energy losses resulting from vibrations and/or rotations.
D. the release of electromagnetic radiation which is not from the nucleus (non-radioactive decay).
12. _______ In the quinine lab experiment, we measured the fluorescence of quinine at a wavelength of approximately 450 nm. What was the approximate wavelength of excitation?
A. 450 nm
C. 550 nm
B. 350 nm
D. any wavelength in the infrared region would work
13. _______ What is the role of the anoxic limestone drain at the passive treatment site at Jennings?
A. To decrease alkalinity while promoting metal precipitation.
B. To decrease alkalinity while minimizing metal precipitation.
C. To increase alkalinity while promoting metal precipitation.
D. To increase alkalinity while minimizing metal precipitation.
14. _______ Which of the following characteristics are indicative of good stream quality?
A. zero turbidity and high dissolved oxygen
B. some turbidity and high dissolved oxygen
C. zero turbidity and low dissolved oxygen
D. some turbidity and low dissolved oxygen
15. _______ In the Kool-Aid lab, the dye eluting from the column first would be best characterized as:
A. the more polar dye; a dye that absorbs red light
B. the more non-polar dye; a dye that absorbs blue light
C. the more polar dye; a dye that absorbs green light
D. the more non-polar dye; a dye that absorbs yellow light
16. _______ For the GC post-lab, we identified the peak at 2.6 min as being the caffeine peak. How did we know that caffeine had a retention time of 2.6 min?
A. The peak at 2.6 min was the only peak in the sample chromatograph, so it had to be the analyte of interest.
B. The peak at 2.6 min was the largest peak in the sample chromatograph, so it had to be the analyte of interest.
C. We were able to compare the standard chromatograph to the sample chromatograph to determine the analyte of interest.
D. We were able to estimate the retention time based on the boiling point of caffeine and the flow rate of the mobile phase.
17. _______ Quantum dots are effective for bio-imaging living tissue because:
A. the fluoresce in the near-IR region, away from aqueous absorption.
B. they fluoresce in the mid-visible region, away from organic molecule absorption.
C. they fluoresce in the far-UV region, away from organic molecule absorption.
D. they fluoresce in the mid-visible region, away from aqueous absorption.
18. _______ In the one article we read, a new compound had been synthesized based on a artemisinin-quinine hybrid. A quinine hybrid was synthesized:
A. to reduce side effects associated with the quinine alone.
B. to increase uptake and efficacy of the active quinine compound.
C. because the malaria parasites are becoming resistant to quinine.
D. All of the above.
19. _______ Supercritical Fluid Chromatography (SFC) is more environmentally friendly than HPLC because:
A. it requires less energy to operate than HPLC.
B. less organic solvent is required for separations.
C. it emits carbon dioxide, a non-greenhouse gas.
D. all of the above
20. _______ Based on the article, microorganisms are important in the abandoned mine drainage process:
A. because they help reduce and thus dissolve metals from the mine tailings.
B. because they help oxidize and thus dissolve metals from the mine tailings.
C. because they help neutralize any acid formed from the mine tailings.
D. because they help neutralize any base formed from the mine tailings.
21. _______ Internal standards can be used as quality control to:
A. assess the accuracy of your calibration curve.
B. asses the recovery of your analyte during sample preparation.
C. assess the precision of your sample replicates.
D. All of the above.
22. _______ In comparison with high performance liquid chromatography (HPLC), gas chromatography offers:
A. larger sample capacity and shorter column lengths.
B. larger sample capacity and longer column lengths.
C. smaller sample capacity and shorter column lengths.
D. smaller sample capacity and longer column lengths.
23. _______ In general, how does an increase in flow rate affect the HPLC separation of two compounds with very similar retention times?
A. Both resolution and efficiency are decreased.
B. Both resolution and efficiency are decreased.
C. Resolution is decreased, but efficiency is increased.
D. Resolution is increased, but efficiency is decreased.
24. _______ Which term in the van Deempter equation is independent of flow rate?
A. The “equilibration” term.
B. The “diffusion” term.
C. The “multiple path” term.
D. None of the above; all are affected by changes in flow rate.
25. _______ A chromatograph in liquid chromatography is a plot of:
A. time versus detector response
B. detector response versus time
C. peak area versus concentration
D. concentration versus peak area
B.
Short Answer. Use your knowledge of chemical analysis to answer each of the following questions. Be complete but succinct in you answer.
26. Using energy diagrams AND words, explain why the process of absorbance occurs at a shorter wavelength than the process of emission for a fluorescent molecule.
27. The flame tests that you performed in lab are theoretically similar to the process utilized by the instrumental method flame atomic emission spectroscopy (AES). Use your knowledge of the flame test to explain the basic principles underlying AES. Based on your understanding of the flame test, describe how AES can be both qualitative and quantitative.
28. Describe the process by which “biological parameters” were used at Jennings to assess stream quality. How does this information complement the “chemical parameters” results?
Bonus: What is of national significance about today’s date, April 18 th ?
Why had Donald Trump been doing a lot of TV interviews recently?








