1. dia
advertisement
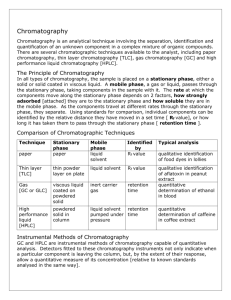
ANALYTICAL SEPARATIONS Precipitation Gravimetry •Precipitation •Filtration •Washing •Drying or ignition •Measuring •Calculation Separating species by distillation Determination of ammonia Determination of carbon dioxide Extraction Distribution between organic and water phase Separation of metal ions as chelates Ions are: soluble in water insoluble in non polar-organic phase Separation of Fe3+ ion O O ) NH4+ (C6H5 NO Cupferron: ionic (C6H5 )3 Fe NO ferric cupferrate: neutral Separating ions by ion exchange Cation exchange: xRSO3-H+ + Mx+ (RSO3-)xMx+ + xH+ solid soln solid soln where: Mx represents a cation and R a part of resin containing sulfonic acid group Anion exchange: xRN(CH3)3+OH- + Ax- [RN(CH3)3]xAx- + xOH- solid soln solid soln where: Ax- represents an anion and R a part of resin containing trimethyl ammonium group After ion exchange cations or anions are on the resin, it should remove them Chromatography Classification of chromatographic methods Stationary phase Mobile phase solid liqiud Gas chromatography GC gas GSC GLC Supercritical chromatography Supercritical fluid SFC SFC TLC IC GPC,SEC PC Normal phase (HPLC-NP) Reversed phase (HPLC-RP) SFC Liquid chromatography LC Liquid chromatography LC liquid liquid CE GEL ELFO Modes of chromatographic separation Frontal chromatography Displacement chromatography Elution chromatography Interactions in chromatography 1. Physical interactions -sorption: adsorption absorption (solvation, distribution) chemisorption -hydrofil-interactions -hydrofob-interactions -interactions based on size exclusion 2. Chemical interactions -acid-base interactions -complex formation -H-bond interactions 3. Biochemical interactions -biochemical affinity The chromatographic process Consequences: •Analytes are moving with different rates (differential migration) •In the course of chromatographic process band are wider and wider (band broadening) Retention data Retention time: tR Dead time: tM (t0) Reduced retention time: tR’ = t R - t M Retention volume: V t F R R where: F, volumetric flow rate (cm3/min) Reduced retention volume: V R t R F t R t M F V R V M The average linear rate of solute migration, (usually cm/s) L tR where L is the length of the column, tR retention time The average linear velocity of the mobile phase molecules, u u L tR The relationship between migration rate and distribution constant The rate as a fraction of the velocity of mobile phase: u fraction of time of solute spend in mobile phase This fraction equals the average numbers of moles of solute in the mobile phase at any instant divided by the total number of moles of solute in the column: u moles of solute in mobile phase total moles of solutes The total number of moles of solute in the mobile phase is : nM = CM x VM in stationary phase: nS = CS x VS Therefore: u C M VM C M VM C S VS u Since distribution constant: KC a A S a A M CS CM therefore: u 1 1 K C VS /VM 1 1 C S VS / C M VM The retention factor: k’ •Time spended of analyte in the stationary phase relating to the mobile phase k’: relative number of moles of analytes in the stationary and mobile phase k’ = nS/nM Other definition of retention factor for analyte A: k A K Vs A where: KA is the distribution constant for analyte A VM Substitution to equation earliers: u 1 1 KA Rearranging: kA tR tM tM L tR L tM 1 1 kA Selectivity factor: kB kA t R 2 t R 1 Always greater than 1.0 Column efficiency and band broadening The plate theory of chromatography One theoretical plate (N): the part of the column, where quasiequilibrium takes place between stationary and mobile phase N 2 tR σt 2 L 2 σL 2 Where: standard deviation and 2 Variance tR 5 , 54 w 2 N 16 tR w 1/ 2 2 w=4 Gauss equation: HETP: Height equivivalent to the theoretical plate (H) H L N The rate theory of chromatography (van Deemter) Porous silica particle particle size (diameter): dP Theory of band broadening 1. Eddy diffusion term (A) multiple path effects C ed p A 2. The longitudal diffusion term (B/u) CdDM u B u 3. Mobile phase mass transfer term (CM/u) CMd 2 p u CM u DM 4. Stationary phase mass transfer term (CS/u) CSMd DM 2 p u CS u The van Deemter equation of chromatography H A B u CM u CSu H u The equation has an optimum (Hopt) where the column efficiency is highest. This optimum has been found at a linear velocity: for gas chromatography at. 0.1 – 0.5 cm/s for liquid chromatography at: 1.0 – 5.0 cm/s At high linear velocities equation can be estimated as: H A B u CSu Resolution Rs t R 2 t R1 1 2 ( w1 w 2 ) Resolution expressed with the terms of plate number, selectivity and retention factors RS 1 4 N2 α 1 k 2' α 1 k 2' Methods to increase resolution RS 1 4 N2 α 1 k 2' α 1 k 2' Effect of increase of retention factor on resolution RS 1 4 How to increase retention factor: •By decreasing eluent strength N2 α 1 k 2' α 1 k 2' Effect of increase of separation factor on resolution RS 1 4 N2 α 1 k 2' α 1 k 2' How to increase separation factor: •By change chemical quality of the mobile phase •By change quality of column Effect of increase of plate number on resolution RS 1 4 N2 α 1 k 2' α 1 k 2' How to increase theoretical plate number: •Decrease of the flow rate (u) •Increase of the column length (L)