Experiment on matter
advertisement

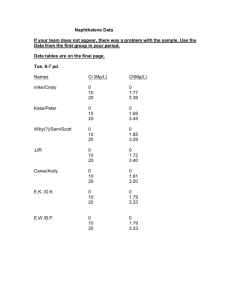
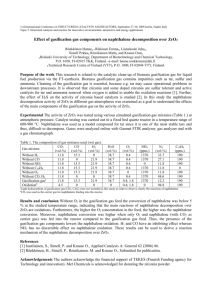
BY ZAINAB BINTI ARIFFIN 90286 •Chemical products should be designed so that at the end of their function they do not persist in the environment and breakdown into innocuous degradation product. •It is better to prevent waste than treat or clean up waste after it is formed. •Substances used in a chemicals process should be chosen as to minimize the potential chemicals accidents. •Study the effects of heating and cooling of the pure substance. •Construct heating and cooling curve of pure substance using experimental data. •Determine the freezing and melting point of pure substance. 200 ml water Naphthalene BOILING TUBE-FILLED WITH NAPHTHALENE. 250ML BEAKER-FILLED WITH 200 ML WATER. BUNSEN BURNER NAPHTHALENE RETORT STAND TRIPOD STAND BOILING TUBE HOLDER THERMOMETER WIRE GAUZE SPATULA WIRE GAUZE WIRE GAUZE 1)Fill one third of the boiling tube with Naphthalene. 2)Place the boiling tube in a beaker of water standing on a gauze and tripod stand over a Bunsen Burner. 3)Heat the water. As the water is heated ,stir the naphthalene. 4) Read and record the temperature every one minute. 5)Repeat steps 3 and 4 until the temperature reaches about 90°C. 6)At every stage,observe and record the state of naphthalene. 7)Take the boiling tube of liquefied naphthalene out of the hot water and allow it to cool slowly. 8)Read and record the temperature at the interval of one minute till it reaches about 70ºC. 9)At every stage, observe the state of the naphthalene. 10)Using the data, draw graphs: »The temperature against time for the heating curve of naphthalene. »The temperature against time for cooling curve of naphthalene. Process Temperature (°C) Heating Around 90.0 Cooling Around 70.0 State Solid become liquid Liquid become solid 1)Describe the change in temperature at the initial stage of heating naphthalene •The temperature increase sharply. Explain. •When the solid is heated, heat energy is absorbed. This causes the particles to gain kinetic energy and vibrate faster. This is why the increased in temperature is observed. 2)When naphthalene is cooled, describe what happens to: 1.The temperature? • The temperature decrease. 2.The state of the naphthalene? • The liquefied naphthalene be in solid state again.