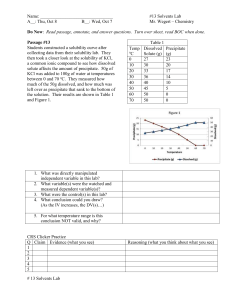
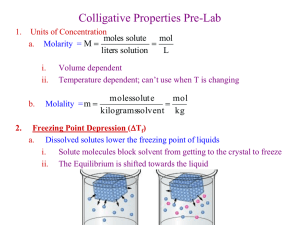
Colligative Properties - Freezing Point Depression
advertisement

Part 1 - Get a Lab Appointment and Install Software: Find the email from your instructor with the URL (link) to sign up at the scheduler. Set up your scheduling system account and schedule your lab appointment. NOTE: You cannot make an appointment until two weeks prior to the start date of this lab assignment. You can get your username and password from your email to schedule within this time frame. Install the Citrix software: – go to http://receiver.citrix.com and click download > accept > run > install (FIRST TIME USING NANSLO). You only have to do this ONCE. Do NOT open it after installing. It will work automatically when you go to your lab. (more info at http://www.wiche.edu/info/nanslo/creative_science/Installing_Citrix_Receiver_Program.pdf) Scheduling Additional Lab Appointments: Get your scheduler account username and password from your email. Go to the URL (link) given to you by your instructor and set up your appointment. (more info at http://www.wiche.edu/nanslo/creative-science-solutions/students-scheduling-labs) Changing Your Scheduled Lab Appointment: Get your scheduler account username and password from your email. Go to http://scheduler.nanslo.org and select the “I am a student” button. Log in to go to the student dashboard and modify your appointment time. (more info at http://www.wiche.edu/nanslo/creative-science-solutions/studentsscheduling-labs) Part 2 – Before Lab Day: Read your lab experiment background and procedure below, pages 1-8. Submit your completed Pre-Lab Questions (page 5-6) per your faculty’s instructions. Watch the Titration Apparatus Control Panel Video Tutorial http://www.wiche.edu/nanslo/lab-tutorials#titration Part 3 – Lab Day Log in to your lab session – 2 options: 1)Retrieve your email from the scheduler with your appointment info or 2) Log in to the student dashboard and join your session by going to http://scheduler.nanslo.org NOTE: You cannot log in to your session before the date and start time of your appointment. Use Internet Explorer or Firefox. Click on the yellow button on the bottom of the screen and follow the instructions to talk to your lab partners and the lab tech Remote Lab Activity SUBJECT SEMESTER: ____________ TITLE OF LAB: Colligative Properties – Freezing Point Depression Lab format: This lab is a remote lab activity. Relationship to theory (if appropriate): In this lab you will explore the effects of dissolved materials on the freezing point of the solvent. Students should have been introduced to the concepts of ionic and molecular compounds and poly-atomic ions. Instructions for Instructors: This protocol is written under an open source CC BY license. You may use the procedure as is or modify as necessary for your class. Be sure to let your students know if they should complete optional exercises in this lab procedure as lab technicians will not know if you want your students to complete optional exercises. Instructions for Students: Read the complete laboratory procedure before coming to lab. Under the experimental sections, complete all pre-lab materials before logging on to the remote lab. Complete data collection sections during your online period, and answer questions in analysis sections after your online period. Your instructor will let you know if you are required to complete any optional exercises in this lab. Remote Resources: Primary – Titration Apparatus; Secondary – Temperature Probe CONTENTS FOR THIS NANSLO LAB ACTIVITY: Learning Objectives........................................................................................................ Background Information ............................................................................................... Estimating Error ............................................................................................................ Pre-lab Questions .......................................................................................................... Equipment ..................................................................................................................... Preparing for this NANSLO Lab Activity ......................................................................... Experimental Procedure ............................................................................................... Exercise 1: Measuring Freezing Points of Various Solutions ....................................... Post-Lab Analysis ........................................................................................................... Creative Commons Licensing ........................................................................................ U.S. Department of Labor Information ......................................................................... 1|Page Last Updated May 26, 2015 2 2-4 4-5 5-6 6 6 7 7 7 8 8 LEARNING OBJECTIVES: After completing this laboratory experiment, you should be able to do the following things: 1. Explain the difference between molecular and ionic substances when they dissolve in a 2. 3. 4. 5. solvent. Use the mathematical expression for freezing point depression to calculate concentration of solute, change in freezing point, etc. Describe various applications for freezing point depression. Determine the value of the van’t Hoff factor for various molecules. Explain the meaning of the van’t Hoff factor. BACKGROUND INFORMATION: The Colligative Properties are a collection of macroscopic phenomena that are related to dissolved solutes in liquid solvents. Solvent: A liquid in which materials are dissolved. Solute: Something that is dissolved into a solvent. They are: freezing point depression, boiling point elevation, vapor pressure lowering and osmotic pressure. These are all properties of bulk liquids that are affected by having dissolved solutes, and they are not dependent on the identity of the solute, but only of the solvent itself. In other words, the Colligative Properties of water are affected in the same way, no matter what you dissolve in the water. All you need to know, in order to calculate the magnitude of the change in boiling point, freezing point, etc., is the identity of the solvent, and the total concentration of the dissolved material. By “total concentration”, we mean the concentration of all the particles that form when the solute dissolves. For example, the molecules of the solute stay intact when a molecular solute such as isopropyl alcohol (also called “rubbing alcohol”) dissolves in water. So, if we dissolved 1.25 moles of isopropyl alcohol in water, there are 1.25 moles of isopropyl alcohol molecules dispersed into the water molecules. However, if we dissolve an ionic solute, such as copper sulfate (sold in hardware stores as “root killer”), each formula unit of the ionic substance breaks into positive and negative ions when it dissolves. In the case of copper sulfate (CuSO4), each formula unit breaks up into a copper ion (Cu2+) and a sulfate ion (SO42-). So if we dissolved 1.25 moles of copper sulfate in water, there would be 2|Page Last Updated May 26, 2015 1.25 moles of Cu2+ and 1.25 moles of SO42- dispersed into the water molecules, or a total of 2.50 moles of ions. Make sure you have memorized the complex ions, like sulfate, phosphate, carbonate, etc. so you can recognize them when you see them in an ionic compound. In the case of ionic compounds, then, the “total concentration” is the concentration of the ionic substance multiplied by the number of ions that it breaks up into when it dissolves. Usually, the number of ions that a substance breaks up into is referred to in this context as the “van’t Hoff Factor” and is given the symbol i. This factor is named for Jacobus van’t Hoff, the first chemist ever awarded the Nobel Prize, which was given for his work on osmotic pressure in 1901 (Nobelprize.org). For a molecular substance, like isopropyl alcohol, the value of i is 1. For an ionic substance, i is equal to the number of ions in each formula unit. For example, the van’t Hoff factor for copper sulfate would be 2.* * It is beyond the scope of this lab activity, but the van’t Hoff factor for some ionic substances is actually not an integer and is less than the theoretical maximum of the number of ions in the formula unit. This is because in some solutions, the ionic substance is not 100% dissociated. However, for this activity, the ionic substances used will be assumed to be 100% dissociated. In this activity, we will be investigating the colligative property of freezing point depression. The relationship between the freezing point and the amount of dissolved solute is expressed mathematically as: ∆𝑇𝑓 = 𝑖 ∙ 𝑚 ∙ 𝐾𝑓 In the above equation, ∆𝑇𝑓 is the change in the freezing point, i is the van’t Hoff factor, m is the concentration of the solute in molality and Kf is the freezing point constant. For water, the value of Kf is 1.86 °C/m. Remember that molality is defined as: 𝑚𝑜𝑙𝑒𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒 𝑘𝑔 𝑜𝑓 𝑠𝑜𝑙𝑣𝑒𝑛𝑡 Notice that in the denominator, it is only the mass of the solvent, not of the entire solution. Why does the freezing point of the solution change just because something is dissolved in the solvent? And why does the freezing point always decrease? To understand this, we have to think about it at a molecular level. When a solvent freezes, the molecules of that solvent must form a particular structure. Think of molecules in a pure liquid phase. The molecules are constantly moving around each other. Even though they are attracted to each other by intermolecular forces, they aren’t in any fixed positions relative to each other. 3|Page Last Updated May 26, 2015 However, when the substance becomes cold enough, the molecular motion slows down enough that the intermolecular forces become significant, and they eventually begin to “stick” together. When the solvent molecules begin to connect with each other, they arrange according to the strongest intermolecular attractions between them and form a particular crystalline arrangement as they become a solid. Of course, a certain amount of energy is required to be removed from the solvent in order to induce this process. Well, if there is something dissolved in the solvent, it interferes with this process. When anything dissolves in a solvent, the solvent molecules form a three-dimensional cage around each of the dissolved particles, whether they are molecules or ions. This makes it much more difficult for the solvent molecules to get into a close arrangement with each other, and more energy must be removed from the system in order to induce the change from liquid to solid. This means that the solution must be made colder than the pure solvent in order to form solid crystals. But it also means that when things are dissolved into the solvent, the motions of the solvent molecules are decreased, because they are “stuck to” the dissolved particles. When the solvent molecules aren’t moving around as much that means the temperature of the system is decreased, because “temperature” is related to how fast the molecules are moving around. The faster the molecular motion, the higher the temperature, and vice versa. There are several practical applications of freezing point depression (Advanced Instruments website). One obvious application that most people are familiar with is the prevention of ice formation in the winter. Salts such as sodium chloride or magnesium chloride are put onto roads and sidewalks, and ice is prevented from forming because the freezing point of the salt/water solution is lower than the freezing point of pure water. A related application that you may have experienced is the use of salt and ice to create a lower temperature for making ice cream. Putting salt onto the ice surrounding the ice cream maker creates a lower temperature than ice alone, and the more salt you add, the colder it gets! ESTIMATING ERROR: You can always estimate the error in any measurement by taking several measurements of it and calculating the standard deviation of the set of results. In other words, if you measure something several times, you are likely to end up with a set of results that are not all exactly the same number. The standard error of that set of numbers is an estimate of how much error there is in your results, and it is equal to the standard deviation divided by the square root of the number of samples in the data set. Here’s an example. Let’s say we measured something five times, and the result was: 4|Page Last Updated May 26, 2015 Trial #1 1 Measurement 0.324 2 0.332 3 0.325 4 0.328 5 0.322 We would report that the value was: average +/- standard error, which in this case is: 0.326 +/- 0.002. This means that the uncertainty in your result (0.326) starts in the third decimal place, because that’s the first digit in your standard error. Since the uncertainty starts in the thousandths place, we have to limit the average value to the thousandths place as well. When reporting uncertainty in this way, you always report only one digit in the standard error. The standard deviation can be calculated manually (you’ve probably done this in a math course), or you can use a spreadsheet program like Excel. If you have fewer than 100 samples to work with, use the “stdev.s” function, which is more accurate for small samples. Sources: Official Web Site of the Nobel Prize, http://www.nobelprize.org/nobel_prizes/chemistry/laureates/1901/ (accessed Oct 3, 2014) Advanced Instruments, Freezing Point Osmometry Practical Applications, http://www.aicompanies.com/index.cfm/AiUniversity/TheoryandBenefitsofFreezingOsmometr y/Freezing_Point_Osmometry_Practical_Applications (accessed Oct 3, 2014) PRE-LAB QUESTIONS: 1. What are the values of the van’t Hoff factor for the following substances? (Hint: It helps to write the chemical formula for each substance.) a. Sucrose ___________ b. Magnesium chloride __________ c. Iron (III) carbonate __________ d. Calcium phosphate __________ e. Sodium chloride ___________ 2. Can the van’t Hoff factor ever be zero? Explain. 3. Calculate the freezing point change from 20.56 grams of magnesium chloride are dissolved in 300.0 grams of water. a. What is the freezing point of this solution? 5|Page Last Updated May 26, 2015 4. Calculate the concentration in molality of calcium phosphate in a solution containing 250.0 grams of pure water when the freezing point is -8.5o C. 5. Complete this sentence: When solid sodium chloride is poured into a glass of water containing ice cubes, the temperature of the water will __________________________. 6. Explain the reasoning behind your answer to question 5. EQUIPMENT: Paper Pencil/pen Computer with Internet access (for the remote laboratory and for data analysis) A spreadsheet program such as Excel PREPARING FOR THIS NANSLO LAB ACTIVITY: Read and understand the information below before you proceed with the lab! Scheduling an Appointment Using the NANSLO Scheduling System Your instructor has reserved a block of time through the NANSLO Scheduling System for you to complete this activity. For more information on how to set up a time to access this NANSLO lab activity, see www.wiche.edu/nanslo/scheduling-software. Students Accessing a NANSLO Lab Activity for the First Time For those accessing a NANSLO laboratory for the first time, you may need to install software on your computer to access the NANSLO lab activity. Use this link for detailed instructions on steps to complete prior to accessing your assigned NANSLO lab activity – www.wiche.edu/nanslo/lab-tutorials. Video Tutorial for RWSL: A short video demonstrating how to use the Remote Web-based Science Lab (RWSL) control panel for the titration apparatus can be viewed at http://www.wiche.edu/nanslo/lab-tutorials#titration. NOTE: Disregard the conference number in this video tutorial. AS SOON AS YOU CONNECT TO THE RWSL CONTROL PANEL: Click on the yellow button at the bottom of the screen (you may need to scroll down to see it). Follow the directions on the pop up window to join the voice conference and talk to your group and the Lab Technician. 6|Page Last Updated May 26, 2015 EXPERIMENTAL PROCEDURE: Read and understand these instructions BEFORE starting the actual lab procedure and collecting data. Feel free to “play around” a little bit and explore the capabilities of the equipment before you start the actual procedure. Once you have logged onto the remote lab, you will perform the following laboratory procedures: EXERCISE 1: Measuring Freezing Points of Various Solutions 1. 2. 3. 4. 5. 6. Measure the temperature of the ice-water solution. Add 100 mL of sucrose solution to the first cup of ice and measure the temperature. Repeat step 2 for the second cup of ice and measure the temperature. Add 100.0 mL of sodium chloride to the third cup of ice and measure the temperature. Repeat step 4 for the second cup of ice and measure the temperature. Return the temperature probe to the first cup (just ice water). As the Lab Technician to dump some sodium chloride into the ice water. As the Lab Technician for the mass of water and the mass of sodium chloride. Measure the temperature. POST-LAB ANALYSIS (Show all necessary calculations. Can be done offline if you run out of time) 1. If you had added more or less glucose and sodium chloride solutions in steps 2 – 5 of the Experimental Procedure, would it have made a difference in the freezing points? Why or why not? 2. When you measure the temperature of water with some ice in it, and if you measure the temperature very close to where the ice cubes are, is that measuring the “freezing point”? Why or why not? 3. Calculate the concentration in motality of the sucrose solution and estimate the error in your result: ___________________molal +/- _________________ 4. Calcuate the concentration in molality of the sodium chloride solution and estimate the error in our result: ____________molal +/- _______________ 5. What happened in step 6 of the exercise? Did it match your hypothesis from the prelab? Explain. 6. Calculate how much sodium chloride actually dissolved in step 6 of the exercise. 7|Page Last Updated May 26, 2015 For more information about NANSLO, visit www.wiche.edu/nanslo. All material produced subject to: Creative Commons Attribution 3.0 United States License 3 This product was funded by a grant awarded by the U.S. Department of Labor’s Employment and Training Administration. The product was created by the grantee and does not necessarily reflect the official position of the U.S. Department of Labor. The Department of Labor makes no guarantees, warranties, or assurances of any kind, express or implied, with respect to such information, including any information on linked sites and including, but not limited to, accuracy of the information or its completeness, timeliness, usefulness, adequacy, continued availability, or ownership. 8|Page Last Updated May 26, 2015