File - Lars Nyberg Math Info
advertisement

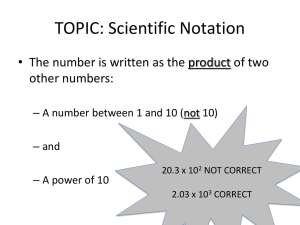
GENERAL EXPONENT RULES AND SCIENTIFIC NOTATION NOTES 8A GENERAL EXPONENT RULES: 32 = −32 = (−3)2 = −(3)2 = (32 ) = SCIENTIFIC NOTATION: A method for expressing, and working with, very large or very small numbers. It is a short hand method for writing numbers, and an easy method for calculations. Numbers in scientific notation are made up of three parts: the coefficient, the base and the exponent. Observe the example below: 5.67 x 105 In order for a number to be in correct scientific notation, the following conditions must be true: 1. The coefficient must be greater than or equal to 1 and less than 10. 2. The base must be 10. 3. The exponent must show the number of decimal places that the decimal needs to be moved to change the number to standard notation. **POSITVE EXPONENT = MOVE THE DECIMAL THAT MANY PLACES TO THE RIGHT. **NEGATIVE EXPONENT = MOVE THE DECIMAL THAT MANY PLACES TO THE LEFT. Changing numbers from scientific notation to standard notation. Ex.1 Change 6.03 x 107 to standard notation. remember, 107 = 10 x 10 x 10 x 10 x 10 x 10 x 10 = 10 000 000 so, 6.03 x 107 = 6.03 x 10 000 000 = 60 300 000 answer = 60 300 000 Instead of finding the value of the base, we can simply move the decimal seven places to the right because the exponent is 7. So, 6.03 x 107 = 60 300 000 Ex.2 Change 5.3 x 10-4 to standard notation. The exponent tells us to move the decimal four places to the left. so, 5.3 x 10-4 = 0.00053 GENERAL EXPONENT RULES AND SCIENTIFIC NOTATION Changing numbers from standard notation to scientific notation NOTES 8A Ex.1 Change 56 760 000 000 to scientific notation Remember, the decimal is at the end of the final zero. The decimal must be moved behind the five to ensure that the coefficient is less than 10, but greater than or equal to one. The coefficient will then read 5.676 The decimal will move 10 places to the left, making the exponent equal to 10. So, 56 760 000 000 = 5.676 x 1010 Ex.2 Change 0.000000902 to scientific notation The decimal must be moved behind the 9 to ensure a proper coefficient. The coefficient will be 9.02 The decimal moves seven spaces to the right, making the exponent -7 So, 0.000000902 = 9.02 x 10-7 Rule for Multiplication – When you multiply numbers with scientific notation, multiply the coefficients together and add the exponents. The base will remain 10. Ex 1. Multiply (3.45 x 107) x (6.25 x 105) 1. First rewrite the problem as: (3.45 x 6.25) x (107 x 105) 2. Then multiply the coefficients and add the exponents: 21.5625 x 1012 3. Then change to correct scientific notation and round to correct significant digits: 2.16 x 1013 NOTE - we add one to the exponent because we moved the decimal one place to the left. Ex. 2. Multiply (2.33 x 10-6) x (8.19 x 103) 1. Rewrite the problem as: (2.33 x 8.19) x (10-6 x 103) 2. Then multiply the coefficients and add the exponents: 19.0827 x 10-3 3. Then change to correct scientific notation and round to correct significant digits 1.91 x 10 -2 Rule for Division – When dividing with scientific notation, divide the coefficients and subtract the exponents. The base will remain 10. Ex. 1 Divide 3.5 x 108 by 6.6 x 104 3.5×108 1. Rewrite the problem as: 6.6×104 2. Divide the coefficients and subtract the exponents to get: 0.530303 x 104 3. Change to correct scientific notation and round to correct significant digits to get: 5.3 x 103