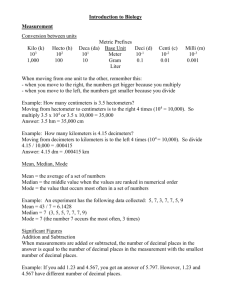
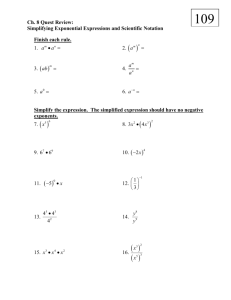
Scientific Notation
advertisement

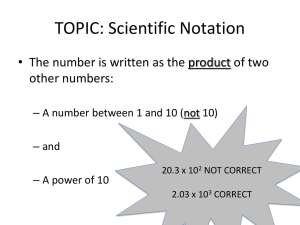
Scientific Notation 1. Why use scientific notation? a. Primarily because physics often deals with very large and very small numbers. b. For example, the distance from the earth to the sun is 150,800,000,000 m and the distance between carbon atoms in diamond is 0.00000000142 m. c. The other reason is that it allows us to write measurements with a precise number of significant figures (More on this later). 2. Numbers greater than 1 a. When a number greater than 1(ex. 150,800,000,000 m) is converted to scientific notation the following rules apply: b. First, move the decimal point until exactly one non-zero digit (a 1-9) appears to the left of the decimal. c. Second, write the new number down while dropping the trailing zeroes. (ex. 1.508) d. Third, write the symbol “x10” after the number. (ex. 1.508 x10) e. Finally, write how many times you moved the decimal as a positive exponent. (ex. 1.508 x1011) 3. Numbers smaller than 1 a. When a number smaller than 1(ex. 0.00000000142 m) is converted to scientific notation the following rules apply: b. First, move the decimal. c. Second, write the new number down while dropping zeroes. (ex. 1.42) d. Third, write the symbol “x10” after the number. (ex. 1.428 x10) e. Finally, write how many times you moved the decimal as a negative exponent. (ex. 1.42 x10-9) 4. A useful mnemonic a. When trying to remember whether to make an exponent positive or negative a mnemonic is useful. b. The correct association in you head should be: (left = positive) and (right = negative). c. Remember this by associating “g = g” from the words “right” and “negative”. 5. Some practice a. b. c. d. e. f. Write the following numbers in scientific notation: 1200 0.005 177,000 0.00000253 8.35