2.1 - Scientific Notation
advertisement

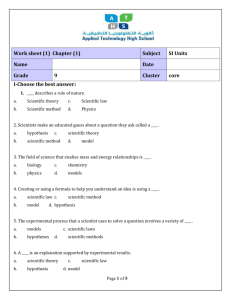
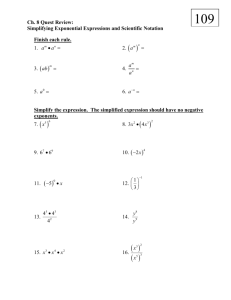
Numbers that are extremely large can be difficult to deal with…sooo Scientists convert these numbers into scientific notation Scientific notation expresses numbers as a multiple of two factors: 1. 2. A number between 1 and 10 (only 1 digit to the left of the decimal!) Ten raised to a power For example: A proton’s mass =0.0000000000000000000000000017262 kg If you put it in scientific notation, the mass of a proton is expressed as 1.7262 x 10-27 kg Remember: When numbers larger than 1 are expressed in scientific notation, the power of ten is positive When numbers smaller than 1 are expressed in scientific notation, the power of ten is negative Try these: Convert 1,392,000 to scientific notation. = 1.392 x 106 Convert 0.000,000,028 to scientific notation. = 2.8 x 10-8 Make sure the exponents are the same!! 7.35 x 102 + 2.43 x 102 = 9.78 x 102 • If the exponents are not the same, you have to make them the same!! • Tip: if you increase the exponent, you decrease the decimal ----- if you decrease the exponent, you increase the decimal • Example: Tokyo pop: 2.70 x 107 Mexico City pop: 15.6 x 106 = 1.56 x 107 Sao Paolo pop: 0.165 x 108 = 1.65 x 107 NOW you can add them together and carry thru the exponent Total= 5.91 x 107 • Multiplication: Multiply decimals and ADD exponents Ex : (1.2 x 106) x (3.0 x 104) = 3.6 x 1010 * Ex: (1.2 x 106) x (3.0 x 10-4) = 3.6 x 102 6 + 4 = 10 6 + (-4) = 2 Division: Divide decimals and SUBTRACT exponents Ex: (5.0 x 108) ÷ (2.5 x 104) = 2.0 x 104 *Ex: (5.0 x 108) ÷ (2.5 x 10-4) = 2.0 x 1012 8–4=4 8 – (-4) = 12 SI units: Systeme Internationale d’ Unites standard units of measurement to be understood by all scientists Base Units: defined unit of measurement that is based on an object or event in the physical world there are 7 base units some familiar quantities are time, length, mass, and temp Time second (s) Many chemical reactions take place in less than a second so scientist often add prefixes, based on multiples of ten, to the base units. ex. Millisecond Length meter (m) A meter is the distance that light travels though a vacuum in 1/299 792 458 of a second. What is a vacuum? Close in length to a yard. Prefixes also apply…ex. millimeter Mass mass is a measurement of matter kilogram (kg) about 2.2 pounds Masses measured in most laboratories are much smaller than a kilogram, so scientists use grams (g) or milligrams (mg). How many grams are in a kilogram? 1000 How many milligrams are in a gram? 1000 Not all quantities are measured in base units A unit that is defined by a combination of base units is called a derived unit. Volume and Density are measured in derived units. Volume The space occupied by an object Unit = cm3 = mL Liters are used to measure the amount of liquid in a container (about the same volume as a quart) Prefixes also applied…ex. milliliter Quantity Base Unit Time Second (s) Length Meter (m) Mass Kilogram (kg) Temperature Kelvin (K) Amount of a substance Mole (mol) Electric current Ampere (A) Luminous intensity Candela (cd) Prefix Symbol Numerical Value in Base Units Power of 10 Equivalent Giga G 1,000,000,000 109 Mega M 1,000,000 106 Kilo K 1000 103 -- -- 1 100 Deci d 0.1 10-1 Centi c 0.01 10-2 Milli m 0.001 10-3 Micro µ 0.000001 10-6 Nano n 0.000000001 10-9 Pico p 0.000000000001 10-12