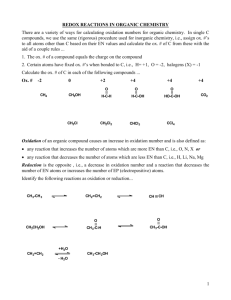
2-Propanol carbon oxidation states
advertisement

You may be wondering… how do I know that going from an alcohol to an aldehyde to a carboxylic acid is oxidation? How do I determine the oxidation state of carbon in an organic molecule? Here is an excerpt from an online resource that might help. This excerpt talks about a ketone being reduced to an alcohol… (IB does not expect you to know how to determine the oxidation number of C in an organic molecule!) OXIDATION STATE To determine the oxidation state of simple inorganic compounds, you assume that the oxidation state of all but one of the elements in the compound is known and determine the remaining element by difference, since the sum of all oxidation number must be zero. For example, in KMnO4, potassium is always +1, each oxygen is -2 (for a total oxidation number of -8) and thus the manganese must be +7. A similar approach is taken in organic compounds, but a carbon is nearly always the unknown. Carbon atoms can have any oxidation state from -4 (e.g. CH4) to +4 (e.g. CCl4). If there is more than one carbon in the molecule, each needs to be calculated, but there are now several more unknown oxidation states than there are knowns to use in their determination. An useful solution to the problem can be generated by accepting two important facts: 1. an absolute value for the oxidation state cannot be determined. 2. only the changes in oxidation state during a reaction are important, so the absolute value isn't needed. The approach described here calculates oxidation state in a consistent manner which always gives the correct oxidation state change for a reaction, and parallels the method used for KMnO4 above. 1. Elements other than carbon are assigned their normal oxidation state: H: +1; O: 2; halogens: -1; and as a consequence OH: -1. There are easily recognized situations where these elements are known to have other oxidation states: hydrides such as LiAlH4 (H: -1), peroxides such as H2O2 (O: -1), or the elements (Cl2, H2, O2: 0). 2. Once the particular carbon of interest is chosen (the one that is changed during the reaction), all other carbons are assigned an oxidation state of 0 (even if you know otherwise)! The oxidation state of the carbon of interest is then calculated by the method used for an inorganic element like Mn in KMnO4 above. Examples: In the reaction below, in which a ketone is converted to an alcohol, the only change in oxidation state occurs at the carbon with the oxygen; the method is outlined and shows that the carbon's oxidation state is reduced from +2 to 0, equivalent to addition of 2 electrons. + Li+ + Al+3 Acetone carbon oxidation states: Carbon number Attached atoms Sum of oxn nos of attached atoms C oxidation state 1 3H, 1C 3(+1) + 1(0) = +3 -3 2 2C, 1O 2(0) + 1(-2) = -2 +2 3 3H, 1C 3(+1) + 1(0) = +3 -3 2-Propanol carbon oxidation states Carbon number Attached atoms Sum of oxn nos of attached atoms C oxidation state 1 3H, 1C 3(+1) + 1(0) = +3 -3 2 2C, 1H, 1OH 2(0) + 1(+1) + 1(-1) = 0 0 3 3H, 1C 3(+1) + 1(0) = +3 -3 The same procedure can be used to determine the oxidation state of nitrogen in organic and inorganic compounds which varies from -3 (NH3) to +5 (HNO3); it occurs in all its oxidation states in organic compounds and it too can participate in redox reactions. http://pages.towson.edu/ladon/orgrxs/reagent/redox.htm