atomic spectra and bohr model
advertisement

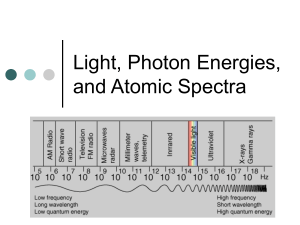
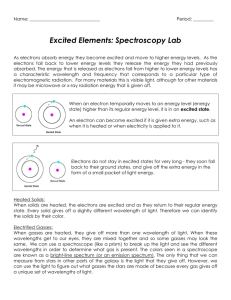
ATOMIC SPECTRA AND THE BOHR MODEL OF THE ATOM Atomic Structure Activity: The Electromagetic Spectrum and Atomic Spectra 1. a) What is the electromagnetic spectrum? The full array of all types of ______________ ______________ is called the electromagnetic spectrum. It extends from the shortest wavelength _____________ rays to the longest wavelength __________waves. *Visible light forms only a _______ portion of the full electromagnetic spectrum. In a vacuum, all EMR travels at the speed of _________ (______________m/s) b) What distinguishes one type of EMR from another? ______________/______________ Wavelength and frequency are _______________ related – as the frequency of the EMR increases, the wavelength ______________ and vice versa. 2. How is the energy of a photon calculated? E = _____ Where: E is the energy in _____________ (J) h is ___________ ____________ (6.6 x 10-34) f is the _________________ (of the EMR) in hertz (Hz) Example: Ultraviolet (UV) light that causes tanning and burning of the skin has a higher energy per photon than infrared (IR) light from a heat lamp. *Calculate the energy of a 1.5 x 1015 Hz UV photon and a 3.3. x 1014 Hz IR photon. energy of UV photon: energy of IR photon: E = hf E = hf = = = = *Therefore, the energy of a photon is solely dependant on the _________________ of the EMR. (i.e. higher frequency = more ______________) (e.g. E of UV photon > E of blue light photon > E of red light photon > E of infrared photon etc.) 3. a) What is a spectroscope? invented by Robert Bunsen and Gustav Kirchoff Their spectroscope was a _________ that split light into its component frequencies. [e.g. A spectroscope splits white light into a __________________ (colours blend into one another) “rainbow” spectrum. Remember: Each colour represents a different frequency/wavelength of EMR. b) What is spectroscopy? a technique for analyzing atomic ________________ (e.g. Stellar spectroscopy is a technique used to identify the _______ present in the atmospheres of distant stars.) 4. What is an emission (____________-line) spectrum? How is it attained? (**ACTIVITY gas discharge tubes) When a gas is excited by __________ or ______________, for example, and then passed through a __________________, a series of bright lines (against a ___________ background) of light are produced. The resulting array of bright lines is called an ___________________spectrum. A bright-line spectrum is not _____________________ (i.e. a complete rainbow of colours is not observed). *View the following ANIMATION: http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/linesp16.swf 5. What is an absorption spectrum (____________-line spectrum)? How is it attained? *Absorption Spectra: http://jersey.uoregon.edu/vlab/elements/Elements.html A cool gas in front of a continuous source of light produces an ________________ spectrum – a series of dark spectral lines (i.e. missing parts) among the colours of the rainbow. This is the type of spectrum observed in stellar (star) spectroscopy. 6. What is the relationship between the bright and dark-line spectrums of an element? The dark lines in an element’s absorption spectrum are in the _________ position as the bright lines in the emission spectrum of the same element. 7. How do the emission/absorption spectrums produced by different atoms compare? Each element produces its own ___________ emission/absorption spectrum. The emission/absorption spectrum of an element can be thought of as its “______________”. This is why stellar spectroscopy is so powerful. (e.g. Although we are unable to travel to distant stars/galaxies, we are able to analyze the light emitted by these objects – the light that hits our telescopes and spectroscopes, that is. The light emitted by our sun produces a spectrum consistent with the “fingerprint” of helium. (“helios” = Greek word for “sun”) *Interestingly, helium was “discovered” on the sun before it was discovered here on Earth. Specific Example: THE EMISSION SPECTRUM OF HYDROGEN *ANIMATION: http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/linesp16.swf *ground state = the _____________ energy state available to an electron (also – state of greatest stability) *excited state = any level _____________the ground state *When an electron moves from the ground state to an excited state, it must ___________ energy. When it moves from an excited state to the ground state, it ___________energy. This release of energy is the basis for ___________ ___________. Example: *When an electron of a hydrogen atom has been excited (in an electrical gas discharge tube, for example) to the third energy level falls to the second energy level, it ________ light with a specific energy. This energy corresponds with a specific wavelength in the electromagnetic spectrum. Specifically, an electron that makes a transition form the 3 rd energy level to the 2nd energy level emits exactly 3.03 x 10-19 joules of energy which corresponds with a photon of ________ light with a wavelength of _____ nm (___ x 10-7 m). λ= hc E *Where h is Planck’s constant (6.6 x 10-34) and c = the speed of light (3.0 x 108 m/s). = = Hydrogen’s Emission Spectrum: The photon of green light = electron transition from level ____ to 2. The photon of blue light = electron transition from level ____ to 2 The photon of violet light = electron transition from level ____ to 2. **Notice that all of these transitions are from a _________ level down to level 2. Transitions down to energy level 2 are referred to as the ____________ series. Are other transitions possible? *The ___________ series = transitions from higher levels down to level 1 These transitions result in the emission of EMR in the _________________ portion of the spectrum. Therefore, the “lines” produced by these transitions ____________ be seen with the naked eye. *The ______________ series = transitions from higher levels down to level 3 These transitions result in the emission of EMR in the ____________ portion of the spectrum. Therefore, these “lines” cannot be seen with the naked eye either. *Electrons dropping back to the lowest energy level (n=1) emit the ______ energy. SUMMARY: **What’s important to remember is that the emission spectrums produced by “excited” atoms extend into ranges of the EMR spectrum that we cannot ___________. As a result, the portion of the emission spectrum that we tend to focus on is the one we can see with the unaided eye. *Line Spectra(Interactive): http://www.rsc.org/Education/Teachers/Resources/Databook/int_electron_energy_hydrogen.htm