solutions_3_13
advertisement

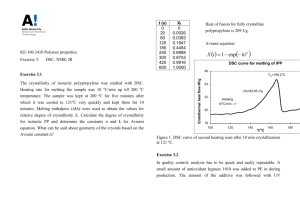
1 KE-100.3410 Polymer properties Exercise 3: DSC, NMR, IR The general Avrami equation is applicable to any type of crystallization. X t 1 exp( kt n ) where k and n are constants typical of the nucleation and growth mechanisms. Derived Avrami exponents for different nucleation and growth mechanisms (Sperling, Introduction to physical polymer science, 4th ed., Wiley & Sons, 2006, pp. 277.): Degree of crystallinity of a polymer can be calculated when melting enthalpy of polymer Hf is measured with DSC and compared with the theoretical fully crystalline melting enthalpy H 0f characteristic for each polymer: X H f H 0f For example for PP H 0f 209 J / g . Exercise 3.1 The crystallinity of isotactic polypropylene was studied with DSC. Heating rate for melting the sample was 10 oC/min up till 200 oC temperature. The sample was kept at 200 oC for five minutes after which it was cooled to 121oC very quickly and kept there for 10 minutes. Melting enthalpies (Hf) were used to obtain the values for relative degree of crystallinity Xt. Calculate the degree of crystallinity for isotactic PP and determine the constants n and k for Avrami equation. What can be said about geometry of the crystals based on the Avrami constant n? 2 t (s) 0 20 60 120 180 240 300 420 600 Xt 0 0.0026 0.0383 0.1947 0.4454 0.6988 0.8753 0.9916 1.0000 Heat of fusion for fully crystalline polypropylene 209 J/g. Avrami equation: X t 1 exp kt n DSC curve for melting of iPP Endothermal heat flow W/g 26 Tm=166,2oC 24 Hf=92,95 J/g 22 Heating 10oC/min --> 20 18 100 120 140 T/oC 160 180 200 Figure 1. DSC curve of second heating scan after 10 min crystallization at 121 oC. Solution 3.1 Degree of crystallinity can be calculated from the measured enthalpy data of isotactic PP Hf and melting enthalpy of completely crystalline PP H 0f : J g X 0.44 0 J H f 209 g H f 92.95 Solving the constants from Avrami equation it needs to be manipulated into the form: 1 X t exp(kt n ) ln(1 X t ) kt n ln( ln(1 X t )) ln k n ln t 3 When ln(-ln(1-Xt)) is plotted as a function of ln t, constant n is obtained from the slope and ln k is the cross point with y-axis. t (s) 0 20 60 120 180 240 300 420 600 Xt 0 0.0026 0.0383 0.1947 0.4454 0.6988 0.8753 0.9916 0.9999 ln t 2.9957 4.0943 4.7875 5.1930 5.4806 5.7038 6.0403 6.3969 ln(-ln(1-Xt)) -5.9555 -3.2420 -1.5299 -0.5284 0.1822 0.7333 1.5644 2.2203 3 1 ln(-ln(1-Xt)) y = 2.4358x - 13.209 -1 -3 -5 -7 3 3.5 4 4.5 5 5.5 6 6.5 ln t From the figure n = 2.44 and ln k =-13.209 k =1.8 10-6 . Based on the Avrami constant n =2.44, the sample would contain two types of crystalline structures, depending on nucleation at least disc-like crystallites. Exercise 3.2 In quality control, analysis has to be quick and easily repeatable. A small amount of antioxidant Irganox 1010 was added to PE in during production. The amount of the additive was followed with UV spectroscopy. For the reference, a set of samples with the known amount of antioxidant (0-0.1 mol-%) was measured: t / cm 0.1 AO 0% A 0.042 AO 0.01% A 0.081 AO 0.05% A 0.29 AO 0.1% A 0.57 4 Use the measurements to formulate a correlation in the form A(c,t) = (kc+a)t, according to Beer-Lambert law that can be used to determine the amount (c) of antioxidant (AO) in the PE sample. The thickness (t) of the sample as well as the UV absorbance (A) is measured. Use the equation then to determine the amount of antioxidant in a PE sample with a thickness of 0.4 cm and UV absorbance A = 0.61. Solution 3.2 Beer Lambert: A kct Use the measured data to construct an equation following the Beer-Lambert law in the form: A(c,t) = (kc+a)t When there is no antioxidant in PE, cAO = 0, t = 0.1 cm, and A = 0.042, we get: A(0, 0.1) (k 0 a) 0.1cm 0.042 a 0.042 0.42cm 1 0.1cm In order to get the constant k we must plot A/t as a function of c so that the starting point for the plot is c = 0, A/t = 0.42 cm-1. A/t 0.42 0.81 2.90 5.70 Determination of constants 6 y = 5205.6x + 0.42 R² = 0.9979 5 A/t (cm-1) c 0 0.0001 0.0005 0.0010 4 3 2 1 0 0 0.0002 0.0004 0.0006 0.0008 0.001 c The slope of the line gives as the constant k = 5206 cm-1. Thus the absorption equation gets the form A(c,t) = (5206c+0.42)t. Solve the equation for c and substitute the values: 5 A 0.61 a 0.42cm1 t 0.4 cm A(c, t ) (kc a)t c 0.00021 k 5206cm1 Thus the antioxidant content of the PE would be 0.021 mol-%. Exercise 3.3 Syndiotactic polystyrene (sPS) crystallizes in various crystal forms () depending on crystallization conditions. The formation of -form is kinetically favorable whereas the formation of -form thermodynamically favorable. Fourier transform spectroscopy (FTIR) is used for analysis of polymer crystallinity. In the FTIR spectrum of syndiotactic polystyrene, characteristic absorbance peaks can be detected; for amorphous (841 cm-1), - (851 cm-1) and -form (858 cm-1). The proportion of - or -crystallinity (Xi) in syndiotactic polystyrene can be determined with the following equations: A851 a X A A A841 851 858 a a A858 a X A A A841 851 858 a a where Ai is the area of each absorbance peak and ai is the absorptivity ratio of each crystal form: a A851 A A841 * 841 a A858 A A841 * 841 A841* is the area of a fully amorphous sample, A841 is the area of the amorphous fraction of a partially -crystalline sample that corresponds to 841 cm-1 absorbance peak and similarly A841 is the area of the amorphous fraction of a partially -crystalline sample that corresponds to 841 cm-1 absorbance peak. 6 The characteristic infrared band of neat s-PS ranging from 940 to 820 cm−1: (a) FTIR spectra of: (a) quenched; (b) coldcrystallized; (c) melt-crystallized s-PS quenched s-PS; (b) melt-crystallized sPS at 240°C for 240 min; (c) sample in thin film sample at 240°C for arbitrary duration using the same s-PS sample. (b) heating scan up to 264°C from 30°C with 10°C/min, then quenched by liquid nitrogen. Wu, H.-D., Wu, S.-C., Wu, I.-D., Chang, F.-C., Novel determination of the crystallinity of syndiotactic polystyrene using FTIR spectrum, Polymer 42 (2001) 4719-25. Three syndiotactic PS samples were analysed by FTIR to determine absorptivity ratios. a) Based on the measurement data calculate the averages for each absorptivity ratio (a, a). b) Three samples of - and -forms were crystallized by using different isothermal crystallization times 2-240 min. Calculate the crystallinity degrees of each sample (Xor X) by using attained absorptivity ratios (a, a). c) Based on crystallinity degrees what can be said of the crystallization of syndiotactic PS? Sample 1 2 3 A841* 1.115 1.240 0.916 A841 0.706 0.842 0.642 A841 0.608 0.742 0.544 A851 0.072 0.071 0.050 A858 0.137 0.134 0.101 7 -crystalline samples: Sample t (min) 1 2 2 10 3 240 A841 1.123 1.086 0.311 A851 0.153 0.225 0.077 A841 0.439 0.448 0.377 A858 0.043 0.066 0.068 -crystalline samples: Sample t (min) 1 2 2 10 3 240 Solution 3.3 a) Determine absorptivity ratios for both - and -forms in each sample. As an example, absorptivity ratio was calculated for the first sample: a ,1 A851,1 A 0.072 0.137 0.176 a ,1 858,1 0.270 A A841,1 1.115 0.706 A841,1 A841,1 1.115 0.608 841,1 The other values are calculated in a similar manner and the average values are calculated as well: Näyte 1 2 3 A841* 1,115 1,240 0,916 A841 0,706 0,842 0,642 A841 0,608 0,742 0,544 A851 0,072 0,071 0,050 A858 0,137 0,134 0,101 ka a 0,176 0,178 0,182 0,179 a 0,270 0,269 0,272 0,270 a= 0.179 ja a= 0.270. b) Taking into account that there is no -form crystalline structure in a sample of -form and vice versa, the degree of crystallinity is calculated: A851,1 0.153 a ,1 0.179 X ,1 0.432 A851,1 0.153 1 . 123 A841,1 0.179 a ,1 8 A858,1 0.043 a ,1 0.270 X ,1 0.266 A858,1 0.043 0.439 A841,1 0.270 a ,1 The other samples of -form are calculated respectively: Sample 1 2 3 t (min) 2 10 240 A841 1,123 1,086 0,311 A851 0,153 0,225 0,077 X 0,432 0,537 0,580 A841 0,439 0,448 0,377 A858 0,043 0,066 0,068 X 0,266 0,353 0,400 and samples of -form: Sample 1 2 3 t (min) 2 10 240 From the result can be seen that crystallinity increases with extending isothermal crystallization period in both crystalline forms. The crystallinity of samples of -form is clearly bigger than the samples of -form. Therefore the crystallization rate of -form is faster. As it was already mentioned the formation of -form is kinetically favorable whereas the formation of -form thermodynamically favorable.