Abrahamsen Richards Cell Biology Plan
advertisement

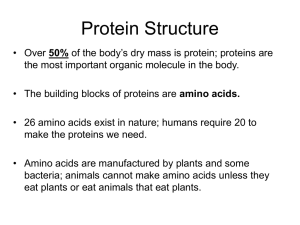
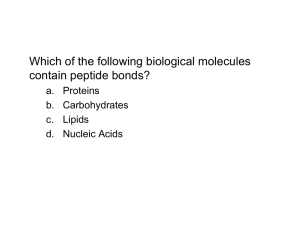
Protein Structure/Function lecture - This is a 2 class-long module at the beginning of the semester. The next two classes are on delta G and kinetics, so the what is presented here lays the groundwork for a 3 week lab that culminates in a major lab write up. Measurable Learning Objectives for this unit: A. List/Describe functions of proteins (knowledge) B. Recognize different structural levels of proteins relate that function (comprehension) C. Compose examples of different protein structural levels (application) D. Analyze how protein structure and function is related to protein chemistry (analysis) E. Recognize and analyze the relationship between similar proteins using protein sequence, domains, and families (comprehension, analysis) F. Explain evolution in cellular context (application) G. Work effectively in groups (skill) H. Communicating scientific ideas in writing (skill) What content do we want them to know? 1) That proteins are made of amino acids strung together in a linear sequence. 2) What an amino acid is 3) That amino acids can be categorized (acidic, basic, uncharged polar, non-polar) 4) What a peptide bond is 5) What primary, secondary, tertiary, quaternary structures are 6) Other molecules in proteins (zinc, iron, chloride) 7) Protein/Protein interactions: quaternary structure 8) How protein function is regulated multiple binding sites phosphorylation nucleotide binding –hydrolysis proteolytic cleavage 9) Protein domains 10) Protein families (evolution, related functions, different structures) ******************************************************* Class Day 1 #1. Warm up questions: (Objective A) What is an amino acid? What functional groups does every amino acid have? What parts of an amino acid can vary? What is a peptide bond? Where does the peptide bond form between two amino acids? How does the peptide bond compare to other kinds of chemical bonds in structure and strength? Where would they get the answers? - From the reading and posted animations and videos #2. Clicker questions for formative assessment of base knowledge (Objective A, B) 1. Which of these is a single amino acid? (Give schematic of primary structure of 3 amino acids linked together) N-C-C-N-C-C-N-C-C R R R 1. C-C-N R 2. N-C-C R 3. C-N-C R 4. C-N-C 2. Which bond is the peptide bond? (Objective B) (label 4 different bonds with arrows on the 1 structure above) 1. A 2. B 3. C 4. D o #3. Lecture info (Objectives A, B) Water-based system (revisit from Bio 190/Organismal Biology pre-req course) Polarity (connection to intro chem and revisit of this material in this context) Bonds that create secondary/tertiary structure Alpha helices, beta sheets, structure/function based on chemistry #4. In Class Activity (Objectives A, B, C, D, E, G) Pipecleaner Protein Activity – The goal is to create a 3D protein with different recognizable chemical regions, and then compare different student-created structures. This is a group activity. Materials: Pipecleaners of different colors, one per student handed out at the beginning of lecture. Each color represents a different aspect of protein chemistry: Red = alpha-helix region Yellow = beta-sheet region White = hydrophobic region Green = region containing a disulfide bond Blue = region containing positively charged amino acids Pink = region containing negatively charged amino acids Steps: 1. Form groups of 5-7 students sitting near each other. 2. Have the students twist pipecleaners together end to end to make a whole protein, creating 1o structure. 3. Have students then fold regions that contain alpha helices and beta sheets into those conformations, creating 2 structure. 4. Have students then create a 3D structure that is consistent with the chemistry: - hydrophobic regions away from the surface (aqueous) environment - positive and negatively charged regions folded to be in proximity o - regions of the same charge folded to show they repel each other - twist the region with a disulfide bond together to indicate a covalent (permanent) bond 5. Have each group examine their own structure (3 ), then compare structures between groups. - Check another group’s protein conformation and make sure you agree with structural/chemical choices o Desired content knowledge outcome: - Realize that different proteins have common elements but can form different looking structures. - Protein structures are stable but flexible #5. Formative Assessment for pipecleaner activity: 1. Discussion: Ask groups about how they decided to make the 3D structure that they chose. Could the structure look different? (Objectives B, D, E) 2. Clicker questions to assess whether they are beginning to apply the basic knowledge: What could happen to the function of this protein if you changed the hydrophobic amino acids in a region of a protein to polar amino acids? (Objective D) A. The protein structure would stay in the same conformation B. The protein structure would stay in a similar conformation, but have small regional differences C. The protein structure would alter in a major way to accomedate the changed chemical groups D. The protein would unwind and lose function What would happen if you heated up this protein and broke hydrogen bonds? (Objective B, D) A. The protein shape would be retained due to the nature of the peptide bond B. The protein shape would be retained due to the noncovalent interactions C. The protein shape would be altered due to the disruption of the noncovalent interactions D. The protein shape would be altered due to the disruption of the peptide bonds. #6. Lecture info: (Objectives A, B, E) Quaternary Structure Other molecules in folding (iron, calcium, zinc, chloride) Homodimers, heterodimers Topics 6, 7 Class Day 2 #7. Warm up Questions (Objective A, B, D) Definition of protein domain Definition of protein families Types of protein regulation strategies Topics 8, 9, 10 - Knowledge based and maybe some comprehension-level questions - derived from the reading, animations What is a protein domain? Name one example of a protein domain. What does it mean when you have two proteins that are in the same protein family? What nucleotides bind to proteins to affect protein function? What is an allosteric binding site? Give an example of a molecule that is an allosteric regulator. #8. Clicker questions (formative assessment) (Objective A, B, D) - Base these on outcomes from Warm up questions #9. Introduce a Summarative Assessment - Protein ID project (Objectives A, D, E, F, G, H) Part A: You have 5 tubes containing 5 different protease proteins, which digest other proteins. However, the tubes are not labeled, and your group’s task is to identify the protease in your tube. What questions do you need to ask about the protein? - the purpose of the assignment is to create a one page document containing molecular and biochemical information on one of 5 proteases that we assign. - this is a group project in two parts: the first part as a group, the second as an individual writing assignment to be included as part of their first Quiz grade. Method: 1. Brainstorm in class: What questions do you have about this protein?What do you need to know in order to understand wha - hopefully this will generate a list of questions related to content covered already (sequence, structure) #10. Lecture: (Objectives A, B, D, E, F) protein domains and protein families Concepts 8,9 #11. Formative Assessment: clicker question (Objectives A, B, D, E, G) You have 3 proteins that bind and store iron. In which of the following ways could you group them? A. Same protein family B. Same protein domain C. Both same protein domain and protein family Think/Pair/Share. Follow up with brief discussion about what additional information you need to help you answer this question. (where are these proteins, what are their functions, etc) #12. Lecture info: (Objectives A, B, D) Protein function regulation Types of regulation Concept 10 #13. Formative Assessment: RSQCC (Objective H, potentially many others depending on their answers) - to be done individually Remember: what are 3 key points you remember about proteins, in rank order (most/less/least) important Summarize: Write a one sentence summary of those 3 key points Question: What is a question that you have about this topic? Connect: for any two of your 3 points, write a sentence on how those two points are connected Comment: Of the time spent on this topic over the last two days, what had the biggest positive impact and why? What had the least impact and why? #14. BIG product - group document (Objectives A, D, E, F, G, H) Part A: You have 5 tubes containing 5 different protease proteins, which digest other proteins. However, the tubes are not labeled, and your group’s task is to identify the protease in your tube. What questions do you need to ask about the protein? (Having already brainstormed, and have asked questions to hopefully guide them to: - what is its function? - how big is it? - is it regulated? if so, how? - where does it function - Related proteins (what protein family does this belong to?) - What domains are within these family members? - what organisms have a version of this protein? Task: As a group, prepare a one page handout of info on this enzyme for your classmates. Post on the Wiki. Part B: (to be done over the next week as we discuss bioenergetics and kinetics) Now look at information sheets from other groups. Compare and contrast your protein to theirs. What questions to you have about these other proteins? - Have them look and evaluate other handouts. #15. Group Discussion, Follow up in class to compare info sheets on proteases. (Objectives A, D, E, F, H) What proteases are similar? What makes them similar? What do you define as similar? What proteases are different? What makes them different? Why are they different? Later the next week as an Individual response: One page written product addressing What did you learn? a. Why is this concept of protein families important? - Why might different versions of proteins exist? Why not have just one? - Why are there different versions between organisms? b. Who cares? c. “I can use this to explain……”