investigating rates of reactions
advertisement

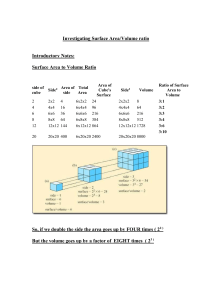
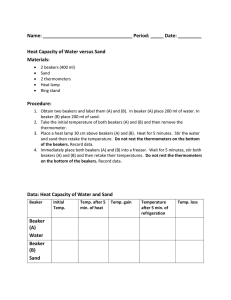
DMAE Chemistry Name: What Factors Affect Reaction Rates? Introduction In the “Discovery Lab” you did, you found that adding yeast significantly increased the rate at which H2O2 decomposed. Yeast contains an enzyme (organic catalyst) that facilitates the reaction. In this investigation, you will explore some other factors that can affect the reaction rate. As you conduct these, keep two things in mind: 1) Why is the reaction rate faster or slower? 2) How does this apply in “everyday life” and your body’s metabolic reactions? The substance used in these investigations is an “effervescent denture tablet.” Although the main purpose is as an antibacterial cleanser for dentures and retainers, it behaves the same as a lot of other substances, so it serves as an effective model for reactions in general. Part 1 Surface Area and Reaction Rate 1) Pour about 30 mL of water into three 50 mL beakers. 2) On one piece of paper, break a tablet into the smallest pieces you can. On another piece, break a tablet into 8 pieces. On a third piece, place a whole tablet. 3) At the same moment (“Countdown 3-2-1-) drop the tablets into the three beaker and record your observations. 4) When the reactions end, rinse the beakers three-four times in a sink. Part 2 Temperature and Reaction Rate 1) Break a tablet into quarters. 2) Pour about 30 mL of cold water into one beaker, 30 mL of room temperature water into the second beaker, and 30 mL of hot water into the third beaker. 3) At the same moment, drop the pieces of the tablets into the three beakers and record your observations. 4) When the reactions end, rinse the beakers three-four times in a sink. Part 3 Acidity and Reaction Rate 1) Pour about 30 mL of soda into one beaker and 30 mL of room temperature water into a second. 2) At the same moment, drop the tablets into the three beakers and record your observations. 3) When the reactions end, rinse the beakers three-four times in a sink. Part 4 Using Technology to Learn More View as many of these as you can: Mark Rosengarten Videos “Rate of Reaction” song (http://www.youtube.com/watch?v=XX9Xo6zm_kM ) “Rate of reactions” video (http://www.youtube.com/watch?v=1tb8n0R2P70&list=PLC9832A956597F2C3&index=78&feat ure=plpp_video) Concentration and reaction rate video (http://www.youtube.com/watch?v=XIkRvRAvaH0) Glow Sticks and Temperature video (http://www.youtube.com/watch?v=fBtCTcRYEUk ) Writing your (individual) Lab Report 1. Purpose Explain three or four important ideas you explore in this investigation. 2. Procedure Describe the important steps you did in each part. 3. Results List what you observed in each part. 4. Math-in-Chemistry Problems (Show all your work.) A. Imagine a cube of salt 1 cm on each side. What is its total surface area? B. Now imagine the salt cube is broken into smaller cubes 1 mm on each side. 1—How many smaller cubes come from the original cube? 2—What is the total area of all the smaller cubes? 5. Discussion Explain what happened in terms of the Collision Theory in each part. Be sure to include the effect of temperature, surface area, acidity, and catalysts. Also, discuss how reaction rates are important within your body’s cells and in manufacturing products. Finally, write about using the videos to learn more. 6. References and acknowledgements Cite in proper form all reading and online resources you used to learn about the topic. If people helped you, give them proper acknowledgements.