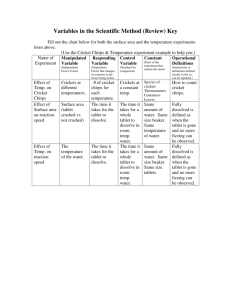
Reaction Rates lab Antacid Race Name___________________________ Experiment 1: Effect of surface area on reaction rate ■ ■ ■ ■ ■ ■ ■ ■ Measure 200 ml of water into each of 2 beakers. Open the Alka Seltzer packet and carefully remove one tablet. Carefully crush the second tablet while still in the packet. Get ready to time the reaction. Put the crushed tablet into a beaker and time the reaction. Observe the reaction and time how long it takes for the reaction to stop for each tablet. (the time for the tablet to be fully dissolved) Place the whole tablet into the other beaker and repeat timing. Record your results Experiment 2: Effect of temperature on reaction rate ■ ■ ■ ■ ■ ■ ■ ■ ■ ■ ■ Measure 200 ml of water into each of 2 beakers. Ask your teacher to heat one of your beakers in the microwave. Place one temperature probe in each beaker. Record the beginning temperature of the water. Open the Alka Seltzer tablet packet. Carefully take out both tablets. Get ready to time the reaction. Place one tablet into the cold water and time the reaction. Place one tablet into the hot water beaker and time the reaction. Observe the reaction and time how long it takes for the reaction to stop. (the time for the tablet to be fully dissolved) Record your results. Record the temperature of each beaker after each reaction was stopped. Observations Before Observations After Experiment 1 Experiment 2 Observation before Observation after Reaction Time Reaction Time Whole Crushed Hot Reaction Time: Cold Reaction Time: Initial Temp: Initial Temp: Final Temp: Final Temp: 1. In experiment 1 the controls were__________________________________ 2. In experiment 1 the variable was___________________________________ 3. In experiment 2 the controls were__________________________________ 4. In experiment 2 the variable was___________________________________ 5. As particle size decreases, reaction rate ____________________________ 6. As temperature increases, reaction rate______________________________ 7. Particle size appears to have _______________ (more or less) of an effect on the rate of reaction than temperature. 8. In experiment 2 what happened to the temperature during the reaction? ______________________________________ Is this an exothermic or endothermic reaction? ___________________________ 9. Think of another way that we could affect the reaction rate in Alka Seltzer tablets. Please provide a detailed explanation of what you could do to test this idea. ( make sure you have a variable and a control) _________________________________________________________________________________________________________ _________________________________________________________________________________________________________ _________________________________________________________________________________________________________ _________________________________________________________________________________________________________ _________________________________________________________________________________________________________ _________________________________________________________________________________________________________ _________________________________________________________________________________________________________ _________________________________________________________________________________________________________ _________________________________________________________________________________________________________ ___________________________________________________________________________ 10. Copy down the class data and graph as Temperature (x-axis) vs. Time (y axis). Class Data Chart Initial Temperature (degrees C) Reaction rate (Time seconds) 11. What can you conclude about temperature and reaction rate from this data? _________________________________________________________________________________________________________ _________________________________________________________________________________________________________ _________________________________________________________________________________________________________ _________________________________________________________________________________________________________ __________________________________________________________________________________________ Graph