Lab Report Lab Participation R 1 2 3 4 R
advertisement

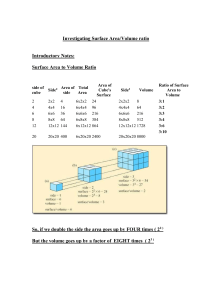
Name: ___________________________ Due Date: ___________________________ Variables that Affect the Rate of Dissolving Lab Lab Report R 1 2 3 4 Lab Participation R 1 2 3 4 Purpose: Understanding factors that affect how quickly a substance dissolves is important in the manufacturing of medicines, dyes, and processed foods. This lab will help you identify factors that affect the rate at which substances dissolve. Materials: • 2 beakers • thermometer • cool water • popsicle stick • warm water • stopwatch • 6 sugar cubes PART A: Changing the temperature of the solvent Hypothesis: ____________________________________________________________ _______________________________________________________________________ Procedure: 1. Make sure you write a hypothesis 4. Add one whole sugar cube to each for this part. Indicate what will beaker. happen, and why it will happen. 5. Stir the beakers at the same speed, 2. Pour 200 mL of tap water into one and record how long it takes for beaker, and 200 mL of hot water each to dissolve. from the kettle into the other. 6. Rinse the beakers. 3. Record the temperature of each beaker. Observations: Beaker Temperature Rate of Dissolving of Solvent (°C) (m:s:ms) 1 (Cool Water) 2 (Warm Water) Pure Substances and Mixtures Observations Page | 1 of 5 Name: ___________________________ Due Date: ___________________________ Conclusions: ____________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ PART B: Changing the Size of the Solute (Salt) Hypothesis: ____________________________________________________________ _______________________________________________________________________ Procedure: 1. Make sure you write a hypothesis 4. Add one crushed sugar cube to the for this part. Indicate what will other beaker. happen, and why it will happen. 5. Stir the beakers at the same speed, 2. Pour 200 mL of tap water in two and record how long it takes for separate beakers. Both water each to dissolve. temperatures should be the same. 6. Rinse the beakers. 3. Add one whole sugar cube to a beaker. Observations: Beaker Size of Solvent (°C) Rate of Dissolving (m:s:ms) Observations 1 Small particles 2 Large particles Conclusions: ____________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ Pure Substances and Mixtures Page | 2 of 5 Name: ___________________________ Due Date: ___________________________ PART C: Rate of Stirring the Mixtures Hypothesis: ____________________________________________________________ _______________________________________________________________________ Procedure: 1. Make sure you write a hypothesis 4. Stir one beaker quickly, and stir for this part. Indicate what will the other beaker slowly. happen, and why it will happen. 5. Record how long it takes for each 2. Put equal amounts of tap water in to dissolve. two separate beakers. Both water 6. Clean and dry the beakers temperatures should be the same. thoroughly. 3. Add one whole sugar cube to each 7. Put away your materials. beaker. Observations: Beaker Stirring the Mixture? 1 Yes 2 No Rate of Dissolving (seconds) Observations Conclusions: ____________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ Pure Substances and Mixtures Page | 3 of 5 Name: ___________________________ Due Date: ___________________________ Analysis Questions 1. List three factors that affect how quickly a solute dissolves in a solvent. • ______________________________________________________________ • ______________________________________________________________ • ______________________________________________________________ 2. Under what 3 conditions does the rate of dissolving increase? _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ 3. When testing the effect of size on dissolving, what other variables need to be controlled? _______________________________________________________________________ _______________________________________________________________________ 4. Explain how you controlled other variables when testing the effect of stirring on dissolving. _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ Pure Substances and Mixtures Page | 4 of 5 Name: ___________________________ Due Date: ___________________________ 5. Use the particle theory to explain how each of the factors (temperature, size, rate of stirring) affects dissolving. Include a sketch in your answer. Pure Substances and Mixtures Page | 5 of 5