Info text #1 - Ice Properties
advertisement

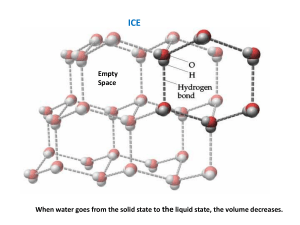
Properties of Ice Melting point Melting point is the temperature at which ice begins to change phase from a solid to a liquid. Salt and other impurities weaken the crystal structure of ice, lowering its melting point. Impure water will freeze at a lower temperature (have a lower freezing point) than pure water. Clarity Clarity describes how clear ice appears when you look through it. Oxygen can be easily dissolved in water, usually through mixing or shaking. When water containing dissolved oxygen freezes, the dissolved oxygen comes out of the water solution, forming tiny bubbles that make the ice cloudy. Dissolved oxygen can be forced out of water by heating it, which is why warm water typically forms clearer ice than cold water. Surface Texture Surface texture describes the smoothness and consistency of the surface of the ice. When water freezes, any precipitation, humidity, or movement of the air above can affect the surface texture of the resulting ice. If the air above the water is moving, the surface may freeze unevenly, leaving an unfrozen hole where an ice spike can form. If the air is humid, the moisture in the air can condense and freeze on the surface of the ice, forming frost. Precipitation can form small frozen droplets or “pebbles” on the surface of ice as well. Cracking and Layering When water is added at different times during the freezing process, each addition freezes separately and is visible as another layer in the ice. Water that freezes unevenly can crack or fracture as parts of it expand or contract at different times.