Answers to book assignment for “Intermolecular Forces” Page 207 5
advertisement

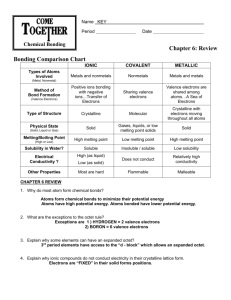
Answers to book assignment for “Intermolecular Forces” Page 207 5. Hydrogen bonding is responsible for water’s high boiling point. In general, boiling point is a measure of the amount of energy needed to overcome intermolecular attractions. Hydrogen bonding is a particularly strong dipole-dipole attraction that causes a powerful attraction between water molecules. This causes water to have a high boiling point. 6. Boiling point is directly correlated with the strength of intermolecular forces. The boiling point is the temperature at which a molecule goes from a liquid state to a gas state. In the gaseous state, all the intermolecular forces are essentially broken. The stronger the intermolecular forces, the more energy needed to break the intermolecular forces, the higher the boiling point. Page 211 37. a. Intermolecular forces are the forces of attraction between molecules. b. Intermolecular forces are weaker than the forces involved in ionic and metallic bonding. c. The strongest intermolecular forces occur between polar molecules. 38. The larger the difference in electronegativities of the atoms in the bond, the greater the polarity of that bond. 39. a. Dipole-dipole forces are the forces of attraction between polar molecules. b. The overall polarity of a molecule depends on the polarity of the individual bonds and the geometry of the molecule. For example, the C-H bonds in methane are polar bonds, but methane as a whole molecule is nonpolar due to its symmetrical geometry. 40. a. An induced dipole is an instantaneous dipole that is produced in a nonpolar molecule when the molecule’s electrons are momentarily attracted by a polar molecule. b. Induced dipoles account tor the solubility of nonpolar compounds, such as oxygen, in polar compounds, such as water. 41. a. Hydrogen bonding is a particularly strong dipole-dipole force that occurs between molecules containing hydrogen atoms and highly electronegative atoms, such as those of N, O, and F. b. Because of the large electronegativity difference between H and F,N, and O, the bonding within molecules containing these atoms is extremely polar. This causes very strong intermolecular forces between these highly polar molecules. 42. London dispersion forces are the weakest of intermolecular forces caused by momentary, induced dipoles created in nonpolar molecules and atoms. 45. a. toward F b. toward Cl c. toward Br d. toward I 46. a. nonpolar b. polar c. polar d. nonpolar 47. a. polar b. nonpolar c. nonpolar d. polar e. polar e. nonpolar f. polar 50. d,c,a,b 52. a. metals and nonmetals b. nonmetals only c. metals only 63. Electronegativity (page 161) Zn 1.6 Br 2.8 S 2.5 Electronegativity (page 161) O 3.5 I 2.5 Cl 3.0 Difference 1.9 0.3 0.5 Bond Type (Page 176) ionic Polar-covalent Polar-covalent More-negative atom O Br Cl 64. 69. F has the highest electronegativity and therefore has the greatest attraction for the electrons it shares in a covalent bond. In other words, it pulls the electrons it shares with other atoms more strongly toward itself. 70. a. Hydrogen can’t be a central atom because in can only form one bond. Also, there are too many valence electrons. b. The carbon and oxygen atoms have more than 8 electrons around them. c. The top chlorine atom has too many electrons. The correct structures are shown below. 71. Because of its symmetry, methane is nonpolar, so the only forces holding one methane molecule to another would be the weak London forces. In contrast, ammonia molecules are highly polar so the attraction between NH3 molecules is much stronger. Therefore, it takes more energy to separate ammonia molecules so that they become a gas.









![QUIZ 2: Week of 09.03.12 Name: [7pts] 1.) Thoughtful list of 3](http://s3.studylib.net/store/data/006619037_1-3340fd6e4f1f4575c6d8cf5f79f0ff3e-300x300.png)
