Atomic Marshmallow Lab In this lab, you are going to be using
advertisement

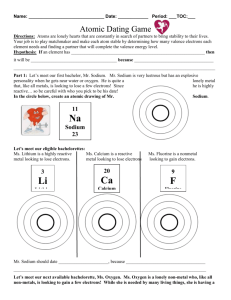
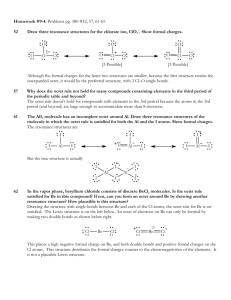
Atomic Marshmallow Lab In this lab, you are going to be using marshmallows to represent what happens when two elements join together to form a compound. You are going to build the element first, and then you will see how the element can share valence electrons (a covalent bond) or give away/take away valence electrons (an ionic bond) to get a set of eight. This is called the octet rule, and when this octet is filled, the atom is stable. All atoms want to get to octet rule stability. Materials 1. Large Marshmallows (these will represent the nucleus) 2. Small Marshmallows (these are your valence electrons) 3. Toothpicks (these are the forces holding the electron to the nucleus) Directions/Protocol/Procedures 1. Build the element Neon (Ne) a. One large marshmallow (nucleus) b. Eight toothpicks (electrostatic force) c. Eight small marshmallows (valence electrons) Question #1: Why does Neon have eight valence electrons? 2. Build the element Sodium (Na) using your materials 3. Now, try to join the sodium with the neon, remembering your octet rule. Question #2: Will the Neon accept Sodium’s valence electron(s)? Why or why not? 4. Set the neon aside. When the experiment is done, you can eat it. 5. Build the element Chlorine (Cl) using your materials 6. Try to join the Chlorine with your Sodium, remembering the octet rule. Question #3: Will the Sodium join up with the Chlorine? Why or why not? 7. Set your sodium and chlorine aside. You will be able to eat them when the experiment is over. 8. Build two hydrogens and one oxygen atom using your materials 9. Join them together to form water Question #4: How do the hydrogens and oxygen come together to form water (you can draw your compound molecule or take a picture)? Is the octet rule satisfied for oxygen? Why is the octet rule not followed for hydrogen? 10. Set your hydrogens aside. 11. Build one more oxygen and one Silicon using your materials. 12. Put them together to form Silicon dioxide Question #5: How do the sulfur and oxygen come together to form Sulfur dioxide (you can draw your compound molecule or take a picture)? Is the octet rule satisfied for oxygen and sulfur? Why or why not? 13. Eat all of your marshmallow atoms or give them away. Question #6: Electronegativity is what determines if a bond is ionic (electrons given/taken) or covalent (electrons shared). Using page 169 in your book, determine what the difference in electronegativity is between your elements. Based on this information, use your book/internet find out what kind of bond your three molecules (NaCl, H2O and SiO2) have. EXTRA CREDIT: Build Methane (CH4), draw or take a picture, and explain how Carbon now has a full octet.