Reaction of Group 7 non metals with metals
Reaction of Group 7 non metals with metals
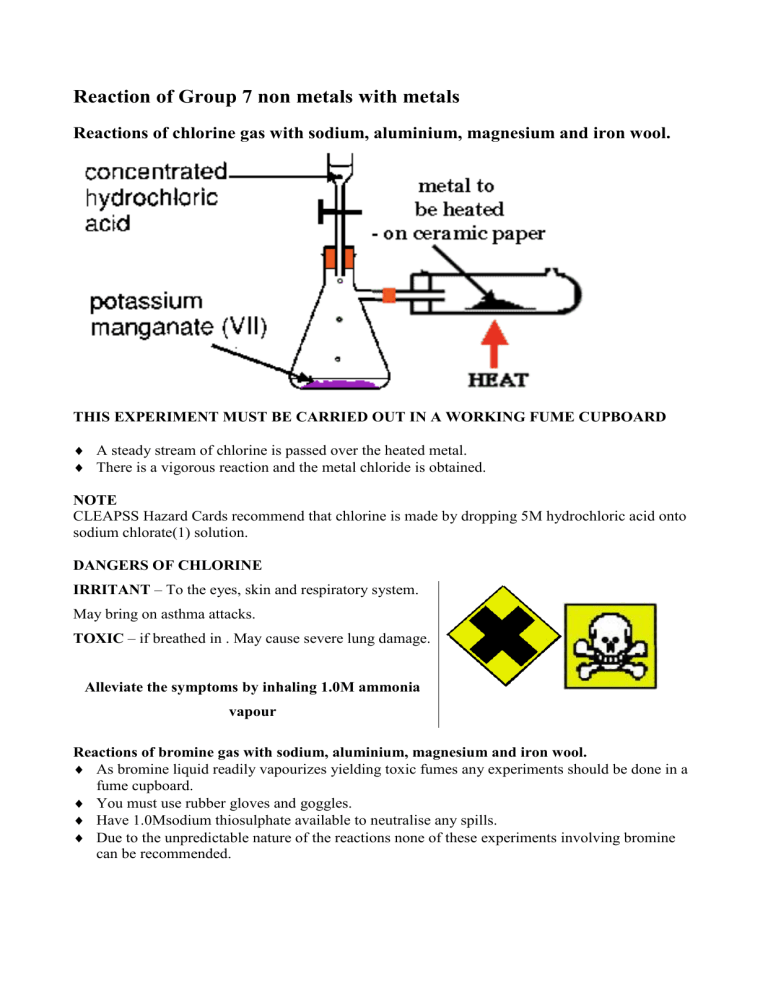
Reactions of chlorine gas with sodium, aluminium, magnesium and iron wool.
THIS EXPERIMENT MUST BE CARRIED OUT IN A WORKING FUME CUPBOARD
A steady stream of chlorine is passed over the heated metal.
There is a vigorous reaction and the metal chloride is obtained.
NOTE
CLEAPSS Hazard Cards recommend that chlorine is made by dropping 5M hydrochloric acid onto sodium chlorate(1) solution.
DANGERS OF CHLORINE
IRRITANT – To the eyes, skin and respiratory system.
May bring on asthma attacks.
TOXIC – if breathed in . May cause severe lung damage.
Alleviate the symptoms by inhaling 1.0M ammonia vapour
Reactions of bromine gas with sodium, aluminium, magnesium and iron wool.
As bromine liquid readily vapourizes yielding toxic fumes any experiments should be done in a fume cupboard.
You must use rubber gloves and goggles.
Have 1.0Msodium thiosulphate available to neutralise any spills.
Due to the unpredictable nature of the reactions none of these experiments involving bromine can be recommended.




![[1] - Boswellsgmt](http://s3.studylib.net/store/data/006603407_1-fadfbce8d94050a9fb3c38a07d86e8ee-300x300.png)






