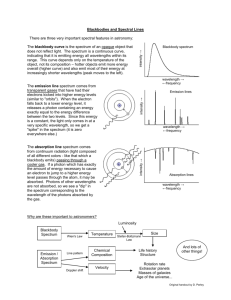
Emission Spectroscopy
advertisement

Part 1 - Get a Lab Appointment and Install Software:
Set up an Account on the Scheduler (FIRST TIME USING NANSLO):
Find the email from your instructor with the URL (link) to sign up at the scheduler.
Set up your scheduling system account and schedule your lab appointment.
NOTE: You cannot make an appointment until two weeks prior to the start date of this lab assignment.
You can get your username and password from your email to schedule within this time frame.
Install the Citrix software: – go to http://receiver.citrix.com and click
download > accept > run > install (FIRST TIME USING NANSLO).
You only have to do this ONCE. Do NOT open it after installing. It will work automatically when you go
to your lab. (more info at
http://www.wiche.edu/info/nanslo/creative_science/Installing_Citrix_Receiver_Program.pdf)
Scheduling Additional Lab Appointments:
Get your scheduler account username and password from your email.
Go to the URL (link) given to you by your instructor and set up your appointment.
(more info at http://www.wiche.edu/nanslo/creative-science-solutions/students-scheduling-labs)
Changing Your Scheduled Lab Appointment:
Get your scheduler account username and password from your email. Go to http://scheduler.nanslo.org
and select the “I am a student” button. Log in to go to the student dashboard and modify your
appointment time. (more info at http://www.wiche.edu/nanslo/creative-science-solutions/studentsscheduling-labs)
Part 2 – Before Lab Day:
Read your lab experiment background and procedure below, pages 1-21.
Submit your completed Pre-Lab Questions (pages 3-4) per your faculty’s instructions.
Watch the Spectrophotometer Control Panel Video Tutorial
http://www.wiche.edu/nanslo/lab-tutorials#emissionspec
Part 3 – Lab Day
Log in to your lab session – 2 options:
1)Retrieve your email from the scheduler with your appointment info or
2) Log in to the student dashboard and join your session by going to http://scheduler.nanslo.org
NOTE: You cannot log in to your session before the date and start time of your appointment. Use
Internet Explorer or Firefox.
Click on the yellow button on the bottom of the screen and follow the instructions to talk to your lab
partners and the lab tech.
Remote Lab Activity
SUBJECT SEMESTER: ____________
TITLE OF LAB: Emission Spectroscopy
Lab format: This lab is a remote lab activity.
Relationship to theory (if appropriate): This activity covers the relationship between colors and
absorbed/emitted light, as well as the relationship between absorption of electromagnetic
radiation and global warming.
Instructions for Instructors: This protocol is written under an open source CC BY license. You
may use the procedure as is or modify as necessary for your class. Be sure to let your students
know if they should complete optional exercises in this lab procedure as lab technicians will not
know if you want your students to complete optional exercise.
Instructions for Students: Read the complete laboratory procedure before coming to lab.
Under the experimental sections, complete all pre-lab materials before logging on to the
remote lab, complete data collection sections during your on-line period, and answer questions
in analysis sections after your on-line period. Your instructor will let you know if you are
required to complete any optional exercises in this lab.
Remote Resources: UV/Vis Spectrometer; Secondary - Emission Tubes.
CONTENTS FOR THIS NANSLO LAB ACTIVITY:
Learning Objectives........................................................................................ 2
Background Information ............................................................................... 2-3
Equipment ..................................................................................................... 3
Pre-lab Assignment ....................................................................................... 3-4
Preparing for this NANSLO lab activity ......................................................... 5
Experimental Procedure ............................................................................... 5-6
Analysis (Can Be Done Offline If Necessary) ................................................. 6-9
Exporting a Graph of the Spectrum .............................................................. 9
Spectrometer NANSLO Control Panel Instructions ....................................... 10-20
Creative Commons Licensing ........................................................................ 21
U.S. Department of Labor Information ......................................................... 21
1|Page
Last Updated May 27, 2015
LEARNING OBJECTIVES:
1. Identify the most intense peaks in the emission spectra for several molecular and atomic
gases.
2. Use the visible emission spectrum for hydrogen gas to determine the electronic
transitions taking place and compare these with the theoretical predictions using the
Rydberg equation.
3. Relate wavelength and color of light.
4. Calculate frequency and energy of electromagnetic radiation from its known
wavelength.
5. Predict absorption of light by a gas from its emission spectrum.
6. Identify an unknown gas by its emission spectrum.
BACKGROUND INFORMATION:
Spectroscopy is the study of the interaction between light and matter, in particular how atoms
or molecules absorb or emit electromagnetic (EM) radiation. Electromagnetic radiation is
characterized by its wavelength or its frequency, which are related by the equation c = λν,
where λ is wavelength (typically units are nanometers for visible light), ν is frequency (units are
s-1, known as Hertz or Hz) and c is the speed of light (299,792,458 m/s). Note that for any wave,
its wavelength times its frequency equals its speed; for electromagnetic radiation that speed is
the speed of light. Also note that as wavelength increases, frequency decreases, and vice versa.
Electromagnetic radiation may be understood as having both wave-like and particle-like
properties, depending on the experimental setup and the type of detector used (in this
experiment, we are measuring wavelengths using a spectrometer that emphasizes the wavelike properties of light). From a particle-like point of view, the smallest amount of EM radiation
(of a particular wavelength) that can be emitted or observed is called a photon. As Albert
Einstein showed more than a century ago, the energy of a photon is proportional to the
frequency of the EM radiation involved (and thus inversely proportional to the wavelength):
Ephoton = hν, where h is called Planck’s constant (h = 6.62607 x 10-34 J•s). Since energy is
conserved and since atoms and molecules almost always emit or absorb one photon at a time,
the change in energy of an atom or molecule will determine the frequency (and wavelength) of
the EM radiation absorbed or emitted: ΔEatom = Ephoton = hν = hc / λ.
Atoms (or molecules) emit photons of very distinctive wavelength (as determined by the
previous equation) when the energy of the atom decreases. Atoms can absorb energy when
they absorb a photon with an appropriate wavelength (a photon whose energy matches the
difference in energy between the initial and final energy states of the atom, ΔEatom). Since
atoms have quantized energy levels (only certain energy levels are possible,) there are only
certain values possible for ΔEatom. Thus, only certain frequencies and wavelengths of EM
radiation will be emitted by the atoms (and molecules) in this experiment.
2|Page
Last Updated May 27, 2015
For most atoms, determining the energy levels possible involves very complex quantum
mechanical calculations. The H atom (hydrogen) is a notable exception since it is the simplest of
atom with only a single electron. Even before quantum mechanics was discovered and
understood, physicists and chemists were able to write a very simple equation to predict the
wavelengths for the EM radiation absorbed or emitted by H atoms known as the Rydberg
equation: 1 / λ = RH {(1/n12) – (1/ n22)} where RH is called the Rydberg constant (0.010967760
nm-1), and n1 and n2 are positive (non-zero) integers called quantum numbers such that n1 <
n2. See your text for more details about the Rydberg equation and to see an example of using
this equation in a calculation.
You may notice that we are using hydrogen molecules (H2) in this experiment whereas the
Rydberg equation only applies to hydrogen atoms (H). Hydrogen atoms are not chemically
stable, but H2 molecules are stable and can be used to fill the glass tube in the hydrogen lamp.
The high-voltage electrical discharge used to power the lamp will temporarily break up H2
molecule into individual H atoms which then gain additional energy from the discharge. This
additional energy is then emitted in the form of visible light as the H atoms return to lower
energy states.
The energy of a photon of electromagnetic radiation is given by the relationship:
E = hν where E = energy in joules, ν = frequency in cycles per second, and h = Planck’s
constant = 6.62607 x 10-34 J•s
The relationship between wavelength and frequency of electromagnetic radiation is:
λν= c where λ = wavelength in meter and c = 2.996 x 108 m/s, the speed of radiant
energy in a vacuum
EQUIPMENT:
Paper
Pencil/Pen
Computer with Internet access
PRE-LAB ASSIGNMENT:
1. Use the Rydberg equation to predict the wavelength of the electromagnetic radiation
emitted for following electron transitions for the hydrogen atom (rounded-off to the
nearest 0.1 nm).
3|Page
Last Updated May 27, 2015
Electron Transition
Predicted Wavelength (𝒏𝒎)
𝑛=3→2
𝑛=4→2
𝑛=5→2
𝑛=4→3
𝑛=2→1
2. Using Figure 1 and your predicted wavelengths above, determine what type of
electromagnetic radiation is produced by these electron transitions in the hydrogen
atom. In other words, does the transition of 𝑛 = 3 → 2 produce ultraviolet (UV), visible
or infrared (IR) radiation? What about the other transitions above?
Figure 1: By Jonathan S. Urie [CC-BY-SA-3.0 (http://creativecommons.org/licenses/bysa/3.0)] via Wikimedia Commons
Electron Transition
𝑛=3→2
𝑛=4→2
𝑛=5→2
𝑛=4→3
𝑛=2→1
Type of Radiation
3. Review the Experimental Procedure below and let your instructor know if you have any
questions.
4|Page
Last Updated May 27, 2015
PREPARING FOR THIS NANSLO LAB ACTIVITY:
Read and understand the information below before you proceed with the lab!
Scheduling an Appointment Using the NANSLO Scheduling System
Your instructor has reserved a block of time through the NANSLO Scheduling System for you to complete
this activity. For more information on how to set up a time to access this NANSLO lab activity, see
www.wiche.edu/nanslo/scheduling-software.
Students Accessing a NANSLO Lab Activity for the First Time
For those accessing a NANSLO laboratory for the first time, you may need to install software on
your computer to access the NANSLO lab activity. Use this link for detailed instructions on
steps to complete prior to accessing your assigned NANSLO lab activity –
www.wiche.edu/nanslo/lab-tutorials.
Video Tutorial for RWSL: A short video demonstrating how to use the Remote Web-based
Science Lab (RWSL) control panel for the air track can be viewed at
http://www.wiche.edu/nanslo/lab-tutorials#emissionspec.
NOTE: Disregard the conference number in this video tutorial.
AS SOON AS YOU CONNECT TO THE RWSL CONTROL PANEL: Click on the yellow button at the
bottom of the screen (you may need to scroll down to see it). Follow the directions on the pop
up window to join the voice conference and talk to your group and the Lab Technician.
EXPERIMENTAL PROCEDURE:
Read and understand these instructions BEFORE starting the actual lab procedure and collecting
data. Feel free to “play around” a little bit and explore the capabilities of the equipment before
you start the actual procedure.
Once you have logged on to the Remote Lab, you will perform the following Laboratory
procedures:
1. Use the control panel to gather data from the emission lamps.
a.
b.
5|Page
Be sure to start the spectrometer so you can view the spectra when the lamps are
energized.
Use the camera to zoom in on each emission lamp to read the labels and
determine what gas is in each one.
Last Updated May 27, 2015
c.
d.
e.
Use the screw-drive robot to position the fiber optic cable for the spectrometer
and record the spectrum of each of the emission lamps.
While viewing each spectrum, use the cursor to find the wavelength of five or six
most intense peaks for each of the gases. Record these in a table.
Also, while each lamp is glowing, zoom in close with the camera and see what
color it appears to be.
ANALYSIS (CAN BE DONE OFFLINE IF NECESSARY):
2. How closely do the first three transitions listed in the table you prepared in the pre-lab
assignment correspond to the observed wavelength of the three largest peaks in the
hydrogen atom emission spectrum?
3. Note that all the most intense peaks for neon gas have wavelengths greater than 580
nm. Based on Figure 2, what colors of visible light are emitted by the neon tube? Does
this explain the apparent color of neon lamps to the ‘naked’ eye?
Figure 2: Visible Portion of EM Spectrum
4. Shown below is a portion of the emission spectrum produced by a mixture of two of the
gases involved in this experiment. Based on your experimental results, does this gas
mixture include helium gas? Explain your reasoning. Can you determine which two gases
are in this gas mixture?
6|Page
Last Updated May 27, 2015
Figure 3: Spectrum of Gas Mixture
5. Iron vapor produces an emission spectrum that includes an intense peak at 527.0 nm.
Determine the frequency (in Hz) for this type of electromagnetic radiation. What color
of visible light corresponds to this wavelength? What is the energy (in J) per photon
emitted at this wavelength? What is this photon energy in units of kJ per mole? (In other
words, one mole of these photons with wavelength of 527.0 nm consists of how many
kilojoules of electromagnetic energy?)
6. The peaks that you observe in the emission spectrum of each gas are also wavelengths
of light that the gas will absorb better than others. So, if a gas shows an emission peak
at 550 nm, the gas will also absorb light with a wavelength of 550 nm. The more intense
the emission peak, the more that light will be absorbed by the gas. Based on this
information, rank the four gases you observed in this experiment in order of how well
they will absorb infrared light. Write the strongest infrared absorber on the left and the
weakest on the right:
Best absorber > next best > next best > worst absorber
7. Based on these results, and the reference listed below, why do you think carbon dioxide
is considered a “greenhouse gas” that we need to be concerned about when compared
to the other gases you observed in this experiment?
Reference: http://www.ecoearth.org/article/Atmospheric_composition
(Bear in mind that these results only take a small portion of the infrared portion of the
spectrum into account.)
7|Page
Last Updated May 27, 2015
8. Figure 4 below shows the absorption spectra of several common gases that are
prevalent in the atmosphere. Peaks in this spectrum indicate the wavelengths that these
gases absorb the best. The horizontal axis is the wavelength in microns which is another
name for micrometers. Note that this horizontal axis is a logarithmic axis. So this single
figure covers a large portion of the electromagnetic spectrum, namely the ultraviolet,
visible and infrared regions of the spectrum. The vertical axis is the percent absorptivity
of each gas (in other words, what percent of EM radiation at a given wavelength is
absorbed by each gas). A percent absorptivity of zero means a gas is completely
transparent at that wavelength. A value of 100 means complete absorbance at that
wavelength so the gas is opaque. The spectrum labeled "Total" corresponds to the
spectrum of the total atmosphere.
a.
The visible spectrum is typically defined as wavelengths between 400 and 700
nm. Convert these wavelength values to microns and locate the visible region in
the figure below. Also locate the ultraviolet and infrared regions of the figure
(hint: does infrared radiation have longer or shorter wavelengths than visible
light?).
b.
Does the spectrum labeled "Total" indicate whether the atmosphere is mostly
transparent or mostly opaque in the visible region? Briefly explain your answer.
c.
The figure also shows that the Earth emits large amounts of “thermal radiation”
at wavelengths between about 5 and 50 microns. Is this in the ultraviolet,
visible, or infrared region of the spectrum? Greenhouse gases can cause global
warming by absorbing this emitted “thermal radiation”, trapping heat in the
Earth’s atmosphere. Are carbon dioxide, water or oxygen (plus a little ozone, O3)
greenhouse gases according to this figure? Briefly explain your answer.
d. Why are scientists and governments much more concerned about carbon dioxide acting
as a greenhouse gas than water or oxygen? What effect do human activities have on the
levels of these gases in the atmosphere?
8|Page
Last Updated May 27, 2015
Figure 4: Solar Radiation
9. Identify the “unknown” gas in the emission lamp labeled “E”. Explain why you think you
are correct.
EXPORTING A GRAPH OF THE SPECTRUM:
Locate the" Export to Clipboard" button. Go to the pull-down box to the right of it and set it to
"Graph Image." Now click the Export to Clipboard button which will place a copy of the
spectrum in your clipboard.
Start a program like Paint or Word or PowerPoint and paste in the spectrum. Save the file with
an appropriate name so you can find it later.
9|Page
Last Updated May 27, 2015
SPECTROMETER NANSLO CONTROL PANEL INSTRUCTIONS
The Remote Web-based Science Lab (RWSL) spectrometer is controlled remotely by using a
web interface as shown below. This NANSLO control panel allows you to control every function
of the spectrometer just as if you were sitting in front of it.
Figure 5: Spectrometer lab interface for emission spectroscopy lab
Communicating with your Lab Partners
As soon as you have accessed this lab interface, click on the “Voice Conference” yellow button
(you may need to scroll down to see it) to view instructions for communicating with your lab
partners and with the Lab Technicians. Only one person can be in control of the equipment at
any one time so talking together on a conference line helps to coordinate control of the
equipment and creates a more collaborative environment for you and your lab partners.
10 | P a g e
Last Updated May 27, 2015
Gaining Control of the Helmholtz Coil Apparatus
Right click anywhere in the grey area of the lab interface and choose “Request Control of VI”
from the dialogue box that appears when multiple students are using the spectrometer at the
same time. After you request control, you may have to wait a short time before you actually
receive control and are able to use the features on this lab interface.
Figure 6: Take control of the lab interface by right clicking
and selecting "Request Control of VI."
Releasing Control of the Emission Spectroscopy Apparatus
To release control of the spectrometer so that another student can use it, right click anywhere
in the grey area of the lab interface and choose "Release Control of VI" from the dialogue box
that appears.
Figure 7: Release control of the lab interface by
right clicking and selecting "Release Control of VI."
11 | P a g e
Last Updated May 27, 2015
Activating the Spectrometer
To activate the spectrometer, click the "Start" button on the far left portion of the lab interface.
The button will now turn yellow and say “Pause”.
Figure 8: After clicking on the "Start" button, it turns to a "Pause" button.
Spectrometer View Window
The Image view Window displays the real-time video feed from a digital camera focused on the
spectrometer, the emission lamps, and a horizontal screw-drive robot with mounted fiber optic
cable located in front of the emission lamps. The four black boxes, labeled A through E and also
labeled from left to right H2, He, Ne, CO2 and ?, are the emission lamps that generate different
colors of visible light depending on the gas that is in them. The optic cable transmits the
detected light to the spectrometer where the spectrum is digitally acquired. The spectrometer
is the small box located near one end of the track.
12 | P a g e
Last Updated May 27, 2015
Figure 9: Spectrometer View Window showing spectrometer,
emission lamps, and robot with mounted optic fiber.
Camera Presets and PAN-TILT-ZOOM Controls
Several camera preset positions have been programmed for use with this lab interface.
Hovering over the grey area where the buttons are will give you a pop-up menu that describes
where each preset is assigned to as shown in Figure 10. Camera presets 1 through 5 allow you
to zoom in quickly to one of the 5 lamps. Preset Camera 6 allows you to see a full view of the
spectrometer, the emission lamps, and a horizontal screw-drive robot with mounted fiber optic
cable located in front of the emission lamps.
13 | P a g e
Last Updated May 27, 2015
Figure 10: Six camera presets.
The four arrows used to pan and tilt allow you to move the camera right to left and up and
down. The two zoom buttons allow you to zoom in to see a closer look at the equipment such
as shown in Figure 11 or zoom out to view more of the room.
Figure 11: Pan, tilt and zoom capabilities.
14 | P a g e
Last Updated May 27, 2015
Setting Up the Spectrometer
To acquire the spectrum of one of the emission lamps, first select Camera Present 6 - Full View
so that you can see the sensor move along the track. Click on the green-colored A button on
the left side of the screen. This will position the sensor in front of this tube and turn on the H2
emission lamp.
Figure 12: Select Camera Present 6 and the "A" button.
Next click on Camera Preset 1 to view a closeup of the H2 emission lamp once it is turned on.
Use the “Nudge Left” and “Nudge Right” buttons to move the fiber optic and make sure you are
getting the maximum signal from the lamp. If the lamp turns off before you are finished, just
click the same letter again, and it will re-energize. By default, the TimeTubeOn is set to 60
seconds. You can adjust this setting; however, because of the high voltage involved in these
lamps, DO NOT set the TimeTubeON field to more than 120 seconds. The lamp status and the
number of seconds it has been energized are shown in the Lamp Status portion of the interface
screen (to the right of the green lamp buttons A – E).
Once you have the signal maximized, click the “Pause” button to hold the image so the light
doesn’t have to be energized while you locate the maximum peaks (Figure 13).
15 | P a g e
Last Updated May 27, 2015
Figure 13: Press the Pause button to "freeze" the spectrum
You will now need to “zoom out” on the spectrum window to view the entire spectrum
properly. Here’s how to zoom in and out on the spectrum:
16 | P a g e
Last Updated May 27, 2015
1. Click on the center button at the lower right of the graph, shown below in Figure 14.
Figure 14: Click button to access other options.
2. This brings up a small sub-menu of other buttons. The only two that are useful to you
are the left-most buttons in the top and bottom rows. Select the left-most button in the
bottom row to view the entire spectrum.
17 | P a g e
Last Updated May 27, 2015
Figure 15: Spectrum Zoom Out Button
3. Select the left-most button in the top row to select specific parts of the spectrum to “zoom
in” on and view more closely (see Figure 16). After clicking this button, you use the mouse
to draw a box around the area that you want to zoom in to. Be sure you draw the box so
that it includes some area past the top of the peak you are interested in or else it will chop
off the top of it in the viewing window. If you accidentally zoom in too far or on the wrong
part of the spectrum, just zoom out and start over again.
18 | P a g e
Last Updated May 27, 2015
Figure 16: Spectrum Zoom In Button
Identifying the Peak to Export
To export a graph, you will first locate the most intense peaks. Make sure you are zoomed out
to view the entire spectrum (Figure 15 above.) Click the button labeled "Enable Cursor" under
the left side of the graph. The green light will come on, and a vertical green cursor line will
appear on the screen. Click the cursor control button. Use the mouse to "grab" the cursor line
by clicking on it and dragging it to the peak that you want to identify. You must have control of
the lab interface to be able to do this activity.
19 | P a g e
Last Updated May 27, 2015
Figure 17: Click the “Enable Cursor” button and then the
“Cursor Control” icon to identify the peak.
There are now two fields under the graph: “Wavelength (nm)” and “Intensity”. The
Wavelength field shows the current position of the cursor, and Intensity shows a relative
intensity reading of wherever the cursor is located (Figure 17).
Use the cursor to find the wavelength of each major peak in the spectrum.
Once you have the cursor on top of a peak, you can zoom in on it to make sure you are really on
the highest part of the peak. (Sometimes they are double peaks!) If you zoom in or out, you will
need to click the cursor control button again in order to move the cursor.
If you want to zoom in on a peak for a closer look, make sure you place the cursor
approximately on that peak before you click the "Spectrum Zoom In" button and draw a bar
around it.
If you lose the cursor while zooming in on peaks, just zoom out again to find it.
20 | P a g e
Last Updated May 27, 2015
For more information about NANSLO, visit www.wiche.edu/nanslo.
All material produced subject to:
Creative Commons Attribution 3.0 United States License 3
This product was funded by a grant awarded by the U.S.
Department of Labor’s Employment and Training Administration.
The product was created by the grantee and does not necessarily
reflect the official position of the U.S. Department of Labor. The
Department of Labor makes no guarantees, warranties, or
assurances of any kind, express or implied, with respect to such
information, including any information on linked sites and
including, but not limited to, accuracy of the information or its
completeness, timeliness, usefulness, adequacy, continued
availability, or ownership.
21 | P a g e
Last Updated May 27, 2015