N14. DETERMINATION OF WATER HARDNESS

N14. DETERMINATION OF WATER HARDNESS
PURPOSE: To determine the total hardness of a saline water sample in mg/L
PROCEDURE
1.
Fill a burette with the standardised 0.01M EDTA
2.
Pipette the sample aliquot required (your teacher will provide you with the volume as the sample changes each session) into three 250 mL conical flasks.
3.
Add purified water to make the volume up to approximately 50mL in the titration flask.
4.
Add 5 mL of the NH
3
/NH
4
Cl buffer and about 50 mg of Erichrome Black T (EBT) indicator powder to each flask
5.
Swirl to dissolve the powder
6.
Titrate to the first permanent appearance of a blue colour.
7.
Repeat until you have three titrations within 0.5 mL.
CALCULATIONS
1.
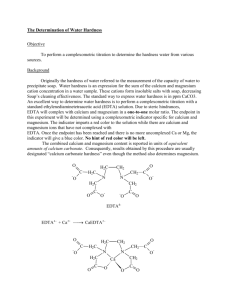
Write the equation for the reaction between Calcium ions and EDTA
2.
Calculate the number of moles of EDTA used in the titration
3.
Determine the number of moles of Calcium ions in your sample aliquot
4.
Calculate the molarity of the solution (for Calcium ions)
5.
Determine the mass of Calcium carbonate in mg/L
QUESTIONS
• What ions make water hard?
• What is the difference between permanent, temporary and total hardness?
• Why is water hardness of concern to so many industries that use hot waer?
• Why does soap fail to work in hard water?
• Explain why it was necessary to filter the sample solution prior to taking the aliquot