Lab 9 - Types of Soilds
advertisement

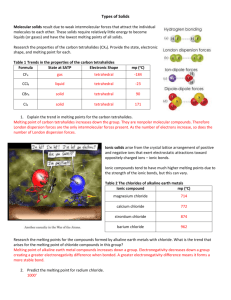
Lab #9 – Properties of Various Types of Solids Name Background There are four main types of solid, each characterized by the two properties listed in the table below: Table #1 Particles occupying the lattice points Bonding force joining particles Ionic Covalent Metallic Macromolecular ions molecules cations in a sea of “orphaned” e-‘s atoms ionic bonds IMF’s metallic bonds covalent bonds In this lab, you will study several properties of solids – melting point, solubility, and conductivity – in the hopes of finding patterns in those properties. You will then be asked to classify several solids as either ionic, covalent, macromolecular, or metallic, based on these properties. Procedure You will test the properties of eight solids. Every group will test samples I – VI, and each group will test a unique set labeled 1A/1B – 12A/12B. Record your observations in the Data Table Melting Point: You will assess the melting point of solids by placing a pea-sized sample in a small test tube, ceramic crucible, or metal crucible and observing the conditions necessary to achieve melting. In addition to observing the conditions in the test tube or crucible, look for evidence of decomposition, sublimation, or the evolution of water. A Fisher burner will be available in the fume hood for generating higher temperatures. Move to the fume hood if fumes are generated during the heating process. Dispose of leftover solids in the waste beaker in the fume hood. REFER to PreLab safety notes before beginning these tests. Temp Range (ºC) Observable conditions at melting point less than 100 Heat gently in small test tube 100 – 300 More aggressive heating needed; no glowing color in test tube glass 300 – 500 Test tube gives orange-yellow color to flame, with increasing color 550 Pyrex test tube begins to soften switch to crucible 550 – 750 Crucible will glow dim red (550ºC) to deep red (750ºC) Solubility: You will test the solubility of each solid in distilled water (polar inorganic), ethanol (polar organic), and hexane (nonpolar organic). Place a pea-sized sample of the solid in a small test tube and add about 2 mL of solvent. Stir aggressively. You will compare the size of the solute pile before and after mixing to assess the degree of solubility. Record your observations in the Data table: Use the terms insoluble, slightly soluble, and soluble to describe each combination. Save each water solution for a conductivity test. Conductivity: Use a multimeter set to one of the resistance settings (Ω) to test the conductivity of the solid, melt, and dissolved form of each solid. Record the approximate resistance in the table. To test the conductivity of a large piece of solid, simply touch the meter electrodes to the sample. To test the conductivity of a granulated or powdered solid, use the penny/washer technique: Sandwich a rubber washer between two pennies, having overfilled the cavity inside the washer with your sample. Use pliers to compress the ‘sandwich’, then touch a probe to each penny. To test the conductivity of a melt, use the probe extensions. Prelab summary Procedural Tips: Heat sample V aggressively after it appears to melt. Safety: Do NOT inhale any fumes. If fumes are generated during heating, move to the fume hood. Heat UK#1A, 2A, 4B, 6A,7A, and 10B in fume hood only. Heat gently. Disposal: Recycle any sample that looks metallic (return to original bottle). All solid and solution waste goes in Waste Beaker in the fume hood. Prelab Questions 1. List the four types of solids studied in this and complete the table below. Type of Solid Particles in lattice Bonding forces in lattice 2. Melting involves weakening the bonds between particles. Considering the Bonding Forces you have listed in the table above, list the four types of solids in order of increasing melting point. 3. In order to conduct electric current, a substance must contain mobile charged partices. Considering that melting and dissolving both render particles mobile, write “cond” in the box for every substance you would expect to be a good conductor of electricity. Solid Melt Solution Covalent Ionic Macromolecular Metallic 4. A substance has a very high melting point, is soluble in polar solvents, and conducts well in the dissolved or molten state. Identify the type of solid, and briefly explain your reasoning. 5. A substance has a very high melting point, is insoluble in all solvents, and does not conduct in any form. Identify the type of solid, and briefly explain your reasoning.