Types of Solids
advertisement

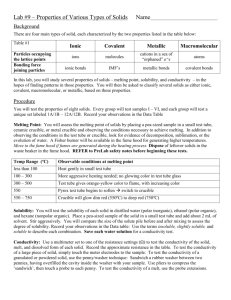
Types of Solids Molecular solids result due to weak intermolecular forces that attract the individual molecules to each other. These solids require relatively little energy to become liquids (or gases) and have the lowest melting points of all solids. Research the properties of the carbon tetrahalides (CX4). Provide the state, electronic shape, and melting point for each. Table 1 Trends in the properties of the carbon tetrahalides Formula State at SATP Electronic Shape mp (oC) CF4 gas tetrahedral -184 CCl4 liquid tetrahedral -23 CBr4 solid tetrahedral 90 CI4 solid tetrahedral 171 1. Explain the trend in melting points for the carbon tetrahalides. Melting point of carbon tetrahalides increases down the group. They are nonpolar molecular compounds. Therefore London dispersion forces are the only intermolecular forces present. As the number of electrons increase, so does the number of London dispersion forces. Ionic solids arise from the crystal lattice arrangement of positive and negative ions that exert electrostatic attractions toward oppositely charged ions – ionic bonds. Ionic compounds tend to have much higher melting points due to the strength of the ionic bonds, but this can vary. Table 2 The chlorides of alkaline earth metals Ionic compound mp (oC) magnesium chloride 714 calcium chloride 772 strontium chloride 874 barium chloride 962 Research the melting points for the compounds formed by alkaline earth metals with chloride. What is the trend that arises for the melting point of chloride compounds in this group? Melting point of alkaline earth metal compounds increases down a group. Electronegativity decreases down a group creating a greater electronegativity difference when bonded. A greater electronegativity difference means it forms a more stable bond. 2. Predict the melting point for radium chloride. 1000+ Metallic solids are held together by an arrangement of metal atoms sharing the electrons of their valence level. The positive nuclei of each metal atom are attracted to the loosely held valence electrons through metallic bonding. View images at http://www.ausetute.com.au/metallic.html. Depending on the attraction of the nuclei to the valence electrons, the strength of metallic bonds will vary, resulting in a wide array of melting points for these solids. Research the alkali metals to observe one of the trends noted in the periodic table. Table 3 Melting points of alkali metals Alkali metal lithium sodium potassium rubidium cesium First ionization energy (kJ/mol) 520 496 419 403 376 mp (oC) 180 98 63 39 28 3. Explain the trend in the melting point for the alkali metals. Decreases down the group because ionization energy decreases. Valence electrons are farther from the nucleus, so the strength of the attractive force decreases creating a weaker bond. 4. The only metal to exist in the liquid state, mercury has one of the broadest temperature ranges as a liquid of any metal. Yet, mercury is unreactive. Research why mercury forms weak metallic bonds. Mercury holds on to its valence electrons tightly because all valence orbitals are full ([Xe]4f145d106s2). This means that electrons cannot flow freely, meaning there is a weak “electron sea” Covalent network solids are the final type of solid using strong covalent bonds to hold the atoms together in large structures (sometimes referred to as macromolecules). The strength of these bonds creates the most stable solids with the highest melting points. The two allotropes of carbon, diamond and graphite, fit into this category. Research the properties of these allotropes. Table 4 The allotropes of carbon Allotrope VSEPR shape around carbon Hybridization of carbon Hardness (Moh’s scale) diamond tetrahedral sp3 10 graphite trigonal planar sp2 1-2 5. Explain the difference(s) in the intramolecular force within diamond and graphite. The 4th carbon bond in graphite is longer and weaker, allowing the layers to slide. The tetrahedral conformation in diamond makes it very strong. 6. Quartz also falls into this category of solids. Research the structure (formula, hybridization, bonding) and properties (hardness, mp, solubility) of this covalent network. SiO2, trigonal and hexagonal crystal structure, Moh’s scale = 7, mp = 1670˚C, insoluble, lustrous