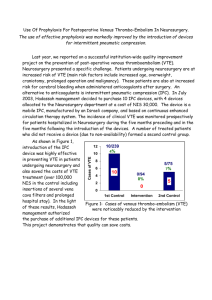
Document 6633113
advertisement

Venous Thromboembolism Prophylaxis in Total Hip and Total Knee Replacement Introduction Venous thromboembolism is the single greatest threat to a patient following joint replacement (Salvati 2000). Despite this, there is a lack of consensus as to which is the optimum form of prophylaxis. The ideal thrombo-prophylaxis should be effective against fatal pulmonary embolism (PE) and have an acceptable level of side effects. Deep vein thrombosis (DVT) is a surrogate marker for fatal PE and is used because it is measureable. However treatments effective against DVT are not necessarily effective against fatal PE. Fatal PE is the appropriate target of prophylaxis. The American College of Chest Physicians (ACCP) were the first group to publish guidelines in 1985 on VTE prophylaxis. The American Academy of Orthopaedic Surgeons (AAOS) set up a working group in 2007 to look at thrombo- embolism prophylaxis. Barrack (2014) summarises nicely the current evidence based position on VTE prophylaxis (Paper included). The current AAOS and ACCP guidelines have converged significantly. The following is the evidence in the literature which you should use when deciding which prophylaxis is appropriate for your patients. ACCP (9th Edition) Summary of Recommendations Recommend use of one of the following (minimum of 10-14 days – 35 days recommended) o LMWH (preferred treatment to other agents) o Fondaparinux o Apixaban o Dabigatran o Rivaroxaban o LDUH (Low dose unfractionated heparin) o VKA (Vitamin K Antagonist) o Aspirin or, o IPCD (Intermittent Pneumatic Compression Device) Recommend starting LMWH 12hrs pre-op rather than 4 hrs, or less pre op AAOS 2012 Summary of Recommendations • Normal Risk ▫ Spinal anaesthetic ▫ Pharmacologic agents and/or mechanical compressive devices. ▫ Early mobilisation ▫ Current evidence unclear which prophylactic strategies are optimal/suboptimal and for what duration of use. • High Risk (previous Hx of DVT) ▫ Pharmacologic agents and mechanical compressive devices. AAOS Recommendations 1. Recommend against routine post-operative duplex US screening. Grade of recommendation: Strong 1 Rationale: Best available evidence is two RCT’s. Found US to be a good “rule in” test for DVT but not a good “rule out” test. “Given the lack of utility of US for diagnosis of unsuspected DVT’s and the lack of any commonly available alternative screening test with greater utility, we do not recommend routine screening”. 2. Patients undergoing elective THR or TKR are already at high risk for VTE. The practitioner might further asses the risk of VTE by determining whether these patients had a previous VTE. Grade of recommendation: Limited Current evidence is not clear about whether factors other than a history of previous venous thromboembolism increase the risk of VTE in patients undergoing elective hip or knee arthroplasty and therefore, we cannot recommend for or against routinely assessing these patients for these factors. Grade of recommendation: Inconclusive 3. Patients should be assessed for known bleeding disorders such as haemophilia and for the presence of known liver disease. Grade of recommendation: Consensus Rationale: The effects of bleeding on functional outcome are significant concern. Evaluating patients for this factors has minimal cost and is low risk to the patient. Current evidence is not clear about whether factors other than the presence of a known bleeding disorder or active liver disease increase the chance of bleeding in these patients and, therefore, we are unable to recommend for or against using them to assess a patient’s risk of bleeding. Grade of recommendation: Inconclusive 4. Patients should discontinue antiplatelet agents before undergoing elective THR or TKR. Grade of recommendation: Moderate Rationale: Preoperative antiplatelet use predicted higher perioperative blood loss. The evidence is not specific to THR or TKR however is still applicable to these patients. 5. We suggest the use of pharmacologic agents and/or mechanical compression devises for the prevention of VTE in patients undergoing elective hip or knee arthroplasty and who are not at an elevated risk beyond that of the surgery itselffor CTE or bleeding. Grade of recommendation: Moderate. Current evidence is unclear which prophylatic strategy (or strategies) is/are optimal or suboptimal. Therefore, we are unable to recommend for or against specific prophylactics in these patients. Grade of recommendation: Inclusive 6. In the absence of available evidence, about how long to employ these prophylactic strategies, it is the opinion of this work group that patients should discuss the duration of prophylaxis with the treating physician. Grade of recommendation: Consensus Rationale: Diversity of clinical opinion regarding the clinical importance of DVT as a surrogate outcome for PE or post thrombotic syndrome. There is moderate evidence to suggest that pharmacological agents and/or mechanical compression devices reduce DVT rates. Evidence is insufficient for extending duration of prophylaxis. 2 In the absence of reliable evidence, it is the opinion of this work group that patients undergoing elective hip or knee arthroplasty, and who have also had a previous venous thromboembolism, receive pharmacologic prophylaxis and mechanical compressive devices. Grade of recommendation: Consensus Rationale: Use of mechanical compressive devices is appropriate, of low risk and consistent with current practice 7. In the absence of reliable evidence, it is the opinion of this work group that patients undergoing elective hip or knee arthroplasty, and who also have a known bleeding disorder (e.g. haemophilia) and /or active liver disease, use mechanical compressive devices for preventing venous thromboembolism. Grade of recommendation: Consensus Rationale: No studies enrolled such patients. The work group deemed that an even greater risk of VTE in these patients justified a consensus based recommendation. 8. In the absence of reliable evidence, it is the opinion of this work group that patients undergo early mobilisation following elective hip or knee arthroplasty. Grade of recommendation: Consensus Rationale: Based on the fact that early mobilisation has minimal cost, is of low risk and is consistent with current practice. 9. We suggest the use of neuraxial anaesthesia for patients undergoing elective hip or knee arthroplasty to help limit blood loss, even though evidence suggests that neuraxial anaesthesia does not affect the the occurrence of VTE . Grade of Recommendation: Moderate Rationale: Studies have shown lower intraoperative blood loss 10. Current evidence does not provide clear guidance about whether inferior vena cava (IVC) filters prevent PEs in patients undergoing elective hip or knee arthroplasty who also have a contraindication to chemoprophylaxis and /or known residual VTE disease. Therefore, we are unable to recommend for or against the use of such filters. Grade of recommendation: Inconclusive Rationale: Limited and conflicting data regarding the benefits of inferior vena cava filters in preventing PE, as well as the fact that no studies included arthroplasty patients. Addendum High Risk Patients with a previous history of VTE, or have thrombophilia, or have active malignancy are at a higher risk of VTE and may require additional prophylaxis. Aspirin We could find no conclusive evidence to say that 150 milligrams (mg) of Aspirin was more effective than 75mg. 75 mg of Aspirin appears to be as effective, when used with Intermittent Pneumatic Compression (IPC). 150mg of Aspirin may increase the risk without increasing the benefit. References Stewart DW and Freshour JE. Aspirin for the Prophylaxis of Venous Thromboembolic Events in Orthopaedic Surgery Patients: A Comparison of the AAOS and the ACCP Guidelines with Review of the Evidence. The Annals of Pharmacotherapy. 2013;47(1):63-74 3 http://aop.sagepub.com/content/47/1/63.abstract?patientinform-links=yes&legid=spaop;47/1/63 The studies looking at Aspirin were heterogeneous in that the doses varied and some weren’t specified. Protein Pump Inhibitors Protein Pump Inhibitors (PPI’s) are recommended in those patients at high risk of gastrointestinal (GI) bleeding or on long term non-steroidal anti-inflammatory (NSAID) use. PPI’s may not be necessary in patients on short-term or post-operative NSAID use. Refernces http://content.onlinejacc.org/article.aspx?articleid=1187955 Recommends the use of PPI’s in patients with high risk of GI bleeding. http://www.med.upenn.edu/gastro/documents/CochranedatabaseNSAIDSpud.pdf A systematic review analysing the effectiveness of PPI’s in the prevention of NSAID induced GI toxicity in patients requiring chronic NSAID use. http://www.nice.org.uk/nicemedia/pdf/CG59NICEguideline.pdf These guidelines discuss the use of PPI’s in patients with OA, requiring prolonged use of NSAIDs. 4