Journal Club RECORD 1 Trial
advertisement

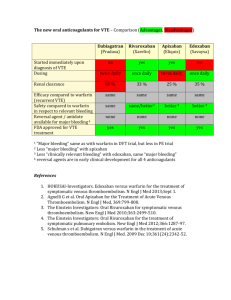
Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Venous Thrombosis and Pulmonary Embolism 1 (RECORD 1 ) Journal Club General Surgery Rotation Background Prophylactic anticoagulation: standard practice after total hip arthroplasty (THA) Minimum recommended duration = 10 days Extended prophylaxis x 5 wks (↓ symptomatic & asymptomatic VTE) > short-term prophylaxis DVT incidence without primary thromboprophylaxis: ~50% of patients undergoing THR If DVT present, fatal PE incidence: 0.1-2.0% Background Rivaroxaban(R): Oral direct inhibitor of factor Xa F=80% Peak: 2.5-4 hours Dose finding studies: 10 mg OD suitable for phase 3 trials Inclusion Criteria > 18 years Scheduled to undergo elective total hip arthroplasty Exclusion Criteria Scheduled to undergo staged, bilateral hip arthroplasty Pregnant or breastfeeding Active bleeding At high risk of bleeding CI for prophylaxis with enoxaparin or a condition that might require an adjusted dose of enoxaparin Exclusion Criteria Conditions preventing bilateral venography Substantial liver disease Severe renal impairment (CrCl < 30 ml/min) Concomitant use of protease inhibitors Planned intermittent pneumatic compression Requirement for anticoagulation that could not be stopped Baseline Characteristics Baseline Characteristics Study Design Randomized, multinational, double-blind R started 6-8 hrs after wound closure Enoxaparin (E) 12 hrs before surgery, restarted 6-8 hrs after wound closure Mandatory bilateral venography the day after the last dose of the study drug (day 36) Follow-up visit 30-35 days after the last dose of the study drug Study Design Sample calculation based on: Assumed rate of 8% for 1° efficacy outcome Noninferiority threshold of 3.5% 1562 patients/ group sufficient to show noninferiority with a power of 95% and type 1 error = 2.5% Per-protocol population of R for 1° efficacy outcome R = 1537, E = 1492 Outcome Measures 1° Efficacy Outcome: Composite of any DVT, non-fatal PE or death from any cause up at 36 days 2 ° Efficacy Outcome: Major VTE Composite of proximal DVT, nonfatal PE or death from VTE Safety Outcome Measures Major bleeding after first dose & up to 2 days after the last dose Major bleeding Fatal bleeding, occurred in a critical organ (e.g. retroperitoneal, intracranial, intraocular, intraspinal) Required reoperation Clinically overt extrasurgical-site bleeding & associated with a fall in Hgb of at least 2 g/dL or requiring blood transfusion of >2 units of PRBC or whole blood Hypothesis Null hypothesis: R inferior to E in per-protocol population If non-inferiority: Superiority analysis (mITT) Absolute margin = 3.5% for 1° efficacy outcome 1.5% for major VTE mITT= Planned surgery done, study drug taken, adequate assessment for thromboembolism Results: Per-protocol Population Outcome Rivaroxaban Enoxaparin ARR 1 efficacy outcome 13/1537 0.8% 50/1492 3.4% 2.5% (1.5-3.6) Major VTE 2/1622 0.1% 29/1604 1.8% 1.7%(1.0-2.4) Results: Incidence of efficacy events (Modified ITT) Outcome Rivaroxaban Enoxaparin ARR P Value 1 efficacy outcome 18/1595 1.1%(0.7-1.8) 58/1558 3.7(2.8-4.8) -2.6 (-3.7 to 1.5) <0.001 Major VTE 4/1686 0.2%(0.1-0.6) 33/1678 2.0(1.4-2.8) -1.7(-2.5 to 1.0) <0.001 Death during ontx 4/1595 0.3%(0.1-0.6) 4/1558 0.3(0.1-0.7) 0.0 (-0.4 to 0.4) 1.00 1/1558 0.1(<0.1 to 0.4) 0.2(-0.1 to 06.) 0.37 Nonfatal PE 4/1595 0.3%(0.1-0.6) Results: Symptomatic VTE Incidence Results: Safety Outcome Event Rivaroxaban (N=2209) Enoxaparin (N=2224) P Value Any on-treatment bleeding 133 (6.0) 131 (5.9) 0.94 Major bleeding 6 (0.3) 2(0.1) 0.18 Non-major bleeding 128 (5.8) 129(5.8) Study withdrawal due to adverse effect 85 (3.8) 100 (4.5) Results: Safety Investigators’ Conclusion Rivaroxaban 10 mg OD > for extended thromboprophylaxis than SC Enoxaparin 40 mg OD Similar number of adverse events Similar safety profiles CASP RCT Checklist Did the study ask a clearly focused question? Yes Was this a randomized controlled trial (RCT) and was it appropriately so? Yes Were participants appropriately allocated to intervention and control groups? Yes Were participants, staff and study personnel ‘blind’ to participants’ study group? Yes CASP RCT Checklist Were all of the participants who entered the trial accounted for at its conclusion? Yes Were the participants in all groups followed up and data collected in the same way? Yes Did the study have enough participants to minimize the play of chance? Yes How are the results presented and what is the main result? Extended thromboprophylaxis with R: very low incidence of thrombosis vs. E with a safety profile similar to that of E CASP RCT Checklist How precise are these results? Definitions of bleed are different amongst different trials 8% outcome expected; found: 3.7% and 1.1% Were all important outcomes considered so the results can be applied? Yes except surgical site bleeding not considered as major bleeding Limitations No details regarding allocation concealment Generalizability of results limited, low # of patients with a previous hx of VTE >than 75 y at extremities of weight Planned THR patients included but not fracture or trauma patients Limitations Patients with severe renal, hepatic failure or at high risk of bleeding excluded Clinically important outcomes not assessed: Post-DVT complications (post thrombotic syndrome) length of hospital stay health related QoL surgical outcomes (infection, wound healing, drainage, range of motion, chronic pain) Limitations Surgical site bleeding excluded from major bleeding events Clinical importance of asymptomatic DVTs as a surrogate measure of symptomatic events not fully elucidated Low incidence of symptomatic VTE, death & major bleeding events: interpret with caution Not powered to investigate differences for these low-frequency events Timing of dose Implications to Practice Rivaroxaban approved and in use No antidote for rivaroxaban More safety data required Needs to be tested in other populations (e.g. fracture, trauma patients) Terminology mITT: any dvt, non-fatal PE, or all cause mortality patients who were considered valid for mITT analysis if they had undergone the appropriate surgery, had taken the study drug, and had an adequate assessment for thromboembolism mITT (major VTE): patients valid for mITT analysis for major VTE if they had undergone the appropriate surgery, had taken the study drug, and had an adequate assessment for thromboembolism in proximal veins Terminology Per-protocol population: Patients who were valid for mITT analysis and had an adequate assessment of thromboembolism with no major protocol deviations Safety population: took at least 1 dose of study drug Symptomatic VTE: safety population of patients who underwent surgery (independent of obtaining evaluable venograms).