emi12675-sup-0001-si
advertisement

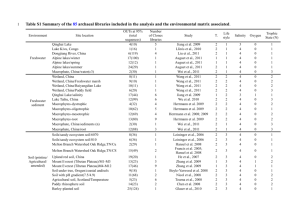
07 Oct 2014 Supporting Information Colonization of rice roots with methanogenic archaea controls photosynthesis-derived CH4 emission Judith Pump, Jennifer Pratscher, Ralf Conrad Experimental Pulse-labeling experiments Pulse-labeling experiments were performed on rice-planted microcosms as described by Pump and Conrad (2014). The microcosms were prepared in plastic pots (1 l volume), which were filled with 0.8 kg air-dried, mechanically ground and sieved (< 2 mm) Italian paddy soil. Alternatively, 80 g sieved soil was amended with 720 g of a mixture (80:1) of quartz sand (Roth, Karlsruhe, Germany) and vermiculite (Thinex New Media, Dortmund, Germany). The pots were flooded with demineralized water, and after one week, each soil microcosm was planted with one fungicide-treated, surface-sterilized and pre-germinated rice seedling (Oryza sativa, var. Koral, type japonica). The planted microcosms were fertilized every second week with half strength Hoagland’s solution (Hoagland and Arnon, 1950) and cultivated in the greenhouse at 25°C, ~ 75% humidity and a photoperiod of 12 h. Demineralized water was added daily to maintain waterlogged conditions. Pulse-labeling experiments were performed at different growth stages of the plants, the early vegetative (2-3 weeks), late vegetative (6-7 weeks), reproductive (9-10 weeks) and ripening (13-14 weeks) stage using duplicate or triplicate microcosms, which were placed in a climate growth cabinet (Conviron, Berlin, Germany) under controlled conditions (25°C, ~ 35 kLux light intensity, photoperiod of 12 h). The microcosms were covered with an acrylic chamber (18 l volume) and pulse-labeled by injection of 170 ml 13CO2 (99 atom% 13C, Sigma-Aldrich, Taufkirchen, Germany) giving an initial CO2 concentration in the headspace of about 1%. Gas samples (1 ml) were taken, and CH4 and CO2 concentrations were analyzed by gas chromatography. The isotopic composition (13C/12C) of CH4 was analyzed using a preconcentration gas chromatograph combustion isotope ratio mass spectrometer system (PreCon-GC-C-IRMS) as described by Brand (1996) and Rice et al. (2001). After 102 h, microcosms were destructively sampled. DNA extraction and molecular analyses The roots from the destructively sampled microcosms were shaken to remove large soil aggregates and loosely adhering soil. The remaining attached soil was sampled with a sterile spatula and used for DNA extraction. The roots were carefully washed, first with tap water and then with sterile demineralized water. Soil and roots were immediately shock-frozen in liquid nitrogen and stored at -80°C until analysis. DNA was extracted from soil or roots using the FastDNA SPIN Kit for Soil (MP Biomedicals) following the manufacturer’s instructions in combination with cell lyses via bead beating (FastPrep-24 Instrument; MP Biomedicals) and chemical removal of humic acids by washing with sterile 5.5 M guanidine thiocyanate. DNA yield and purity were measured using a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Schwerte, Germany). Extracted DNA was stored at -20°C until further processing. Gene copies of archaeal 16S rRNA and mcrA were quantified in duplicate by qPCR using an iCycler iQ thermocycler (Bio-Rad) and the thermal program modified from Lueders et al. (2004). The qPCR conditions targeting archaeal 16S rRNA genes included SYBR Green JumpStart Taq Ready Mix containing 1.25 U Taq DNA polymerase and 0.2 mM dNTP, 3 mM MgCl2 (Sigma-Aldrich), 0.8 µg µl-1 BSA (Roche), 1:1,000 Fluorescein Calibration Dye (BioRad Laboratories GmbH, Munich, Germany), 0.66 µM of each primer 364F (Burggraf et al., 1997) and 934b (Grosskopf et al., 1998a). Similarly, mcrA genes were quantified, but using 3.5 mM MgCl2 and 0.25 µM of each primer mlas and mcrA-rev (Steinberg and Regan, 2008). Archaeal 16S rRNA and mcrA gene amplicons of Methanosarcina barkeri with known copy numbers of the respective target gene were used as qPCR standards in duplicate dilution series for construction of calibration curves in each reaction. For T-RFLP analyses, partial archaeal 16S rRNA genes were amplified using the forward primer A109f and the reverse primer A934b, which was fluorescently-labeled with FAM (6-carboxyfluorescein) at the 5’-terminus (Grosskopf et al., 1998b). Methanogenspecific mcrA genes were amplified using the FAM-labeled forward primer mlas and the reverse primer mcrA-rev (Steinberg and Regan, 2008). 16S rRNA and mcrA gene amplicons were purified with the MinElute PCR Purification Kit (Qiagen GmbH, Hilden, Germany), digested with TaqI and FastDigest Sau96I (Fermentas), respectively, and post-cleaned using SigmaSpin Post-Reaction Purification Columns (Sigma-Aldrich) following the manufacturer's instructions. Cleaned, digested amplicons (2 µl) were mixed with 11 µl of HiDi formamide (Applied Biosystems, Darmstadt, Germany) and 0.3 µl of MapMarker 1000 (BioVentures, Murfreesboro, USA). After denaturation at 95°C for 3 min, fluorescently labeled terminal restriction fragments (T-RFs) were size separated on an ABI 3130 automated sequencer (Applied Biosystems). T-RFLP electropherograms were analyzed using GeneMapper Software Version 4.0 (Applied Biosystems) by peak area integration of each single T-RF. TRFLP profiles were processed as described by Lueders and Friedrich (2003). Clone libraries were constructed after PCR amplification of archaeal 16S rRNA and mcrA genes using primers without FAM-label. The purified gene amplicons were cloned using the pGEM-T Vector System cloning kit (Promega). Randomly selected clones were commercially sequenced from both ends (GATC Biotech AG, Konstanz, Germany) using the primers T7f and M13r targeting flanking regions of the insert. Clone sequences were imported into the ARB software package (Ludwig et al., 2004) for quality check, alignment and phylogenetic tree construction. Phylogenetic analysis of the sequences were done using the SILVA 108 reference dataset for archaeal 16S rRNA and a custom generated reference dataset with reference sequences from NCBI for mcrA sequences. All clone sequences from this study were deposited at GenBank (http://www.ncbi.nlm.nih.gov) under the accession numbers KM273476 - KM273781 and KM273264 - KM273475 (16S rRNA and mcrA, respectively). T-RFs were assigned to phylogenetic groups by using the restriction sites detected in the clone sequences (Table 1), by using assignments described in the literature (Chin et al., 1999; Chin et al., 2004; Kemnitz et al., 2004; Liu et al., 2012; Peng et al., 2008), and by screening mcrA public data bases for Sau96I restriction sites. T-RFs with 465 bp length have no restriction site and thus, cannot be assigned unambiguously. Reference List Brand, W. A. (1996) High precision isotope ratio monitoring techniques in mass spectrometry. J. Mass Spectrometry 31: 225-235. Burggraf, S., Huber, H., and Stetter, K. O. (1997) Reclassification of the crenarchaeal orders and families in accordance with 16S rRNA sequence data. Int. J. Syst. Bacteriol. 47: 657660. Chin, K. J., Lueders, T., Friedrich, M. W., Klose, M., and Conrad, R. (2004) Archaeal community structure and pathway of methane formation on rice roots. Microb. Ecol. 47: 59-67. Chin, K. J., Lukow, T., and Conrad, R. (1999) Effect of temperature on structure and function of the methanogenic archaeal community in an anoxic rice field soil. Appl. Environ. Microbiol. 65: 2341-2349. Grosskopf, R., Janssen, P. H., and Liesack, W. (1998a) Diversity and structure of the methanogenic community in anoxic rice paddy soil microcosms as examined by cultivation and direct 16S rRNA gene sequence retrieval. Appl. Environ. Microbiol. 64: 960-969. Grosskopf, R., Janssen, P. H., and Liesack, W. (1998b) Diversity and structure of the methanogenic community in anoxic rice paddy soil microcosms as examined by cultivation and direct 16S rRNA gene sequence retrieval. Appl. Environ. Microbiol. 64: 960-969. Hoagland, D. R. and Arnon, D. I. (1950) The water-culture method for growing plants without soil. Calif. Agric. Exp. Stat. Circ. 347: 1-32. Kemnitz, D., Chin, K. J., Bodelier, P., and Conrad, R. (2004) Community analysis of methanogenic archaea within a riparian flooding gradient. Environ. Microbiol. 6: 449-461. Liu, G. C., Tokida, T., Matsunami, T., Nakamura, H., Okada, M., Sameshima, R. et al. (2012) Microbial community composition controls the effects of climate change on methane emission from rice paddies. Environ. Microbiol. Reports 4: 648-654. Ludwig, W., Strunk, O., Westram, R., Richter, L., Meier, H., Yadhukumar et al. (2004) ARB: a software environment for sequence data. Nucleic Acids Res. 32: 1363-1371. Lueders, T. and Friedrich, M. W. (2003) Evaluation of PCR amplification bias by terminal restriction fragment length polymorphism analysis of small-subunit rRNA and mcrA genes by using defined template mixtures of methanogenic pure cultures and soil DNA extracts. Appl. Environ. Microbiol. 69: 320-326. Lueders, T., Manefield, M., and Friedrich, M. W. (2004) Enhanced sensitivity of DNA- and rRNA-based stable isotope probing by fractionation and quantitative analysis of isopycnic centrifugation gradients. Environ. Microbiol. 6: 73-78. Peng, J. J., Lü, Z., Rui, J., and Lu, Y. H. (2008) Dynamics of the methanogenic archaeal community during plant residue decomposition in an anoxic rice field soil. Appl. Environ. Microbiol. 74: 2894-2901. Pump, J. and Conrad, R. (2014) Rice biomass production and carbon cycling in 13CO2 pulselabeled microcosms with different soils under submerged conditions. Plant & Soil in press: -doi:10.1007/s11104-014-2201-y. Rice, A. L., Gotoh, A. A., Ajie, H. O., and Tyler, S. C. (2001) High-precision continuousflow measurement of 13C and D of atmospheric CH4. Anal. Chem. 73: 4104-4110. Steinberg, L. M. and Regan, J. M. (2008) Phylogenetic comparison of the methanogenic communities from an acidic, oligotrophic fen and an anaerobic digester treating municipal wastewater sludge. Appl. Environ. Microbiol. 74: 6663-6671. Table S1. Assignment of terminal restriction fragments (T-RFs) to phylogenetic groups based on 266 archaeal 16S rRNA gene and 193 mcrA sequences. Marker genes Phylogenetic affiliation Terminal restriction fragment length (bp) Number of clones Archaeal 16S rRNA Crenarchaeota > 700 32 Nitrososphaera (Thaumarchaeota) 737, 186 89 Methanocellales 391 35 Methanosaetaceae 282 47 Methanosarcinaceae 491, 184 50 Methanobacteriaceae 87, 89, 91 13 Methanosarcinaceae 290, 357, 390, 457 59 Methanobacteriales 369, 372, 434, 438, (465) 38 Methanosaetaceae 110, 220, 384 61 Methanocellales 201, 403 35 mcrA