AspirinSynthesis
advertisement
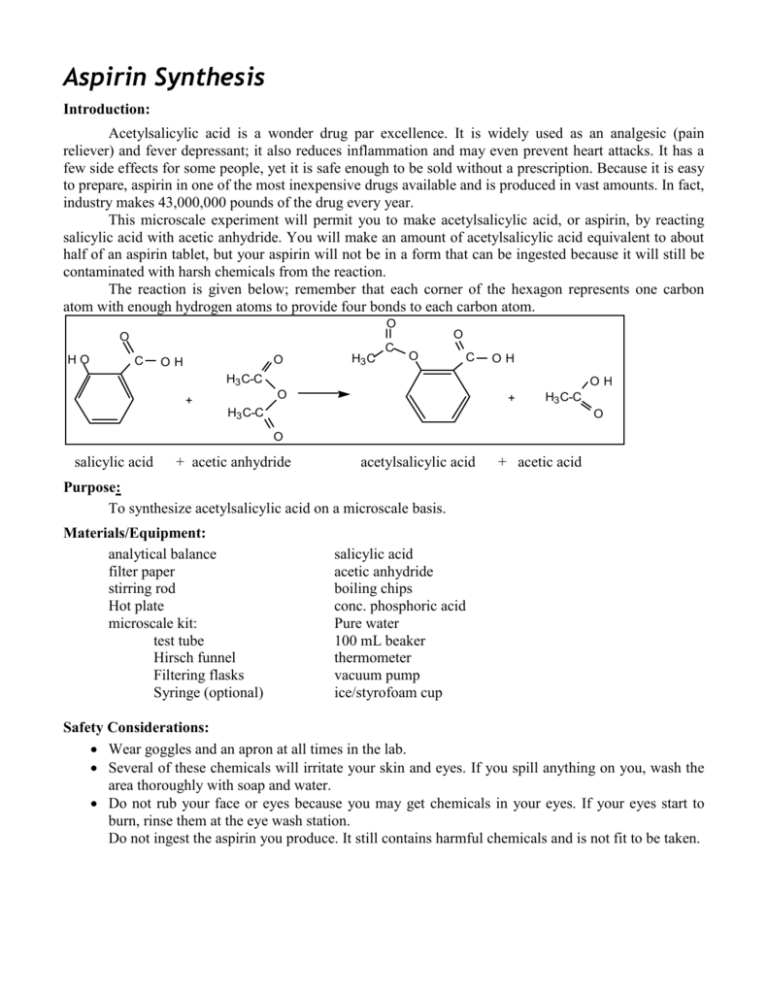
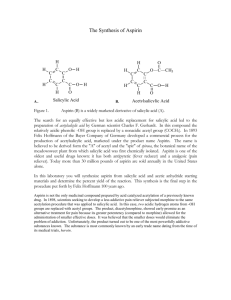
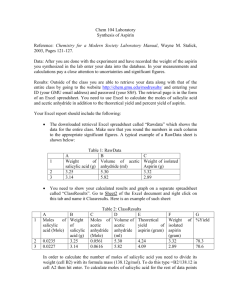
Aspirin Synthesis Introduction: Acetylsalicylic acid is a wonder drug par excellence. It is widely used as an analgesic (pain reliever) and fever depressant; it also reduces inflammation and may even prevent heart attacks. It has a few side effects for some people, yet it is safe enough to be sold without a prescription. Because it is easy to prepare, aspirin in one of the most inexpensive drugs available and is produced in vast amounts. In fact, industry makes 43,000,000 pounds of the drug every year. This microscale experiment will permit you to make acetylsalicylic acid, or aspirin, by reacting salicylic acid with acetic anhydride. You will make an amount of acetylsalicylic acid equivalent to about half of an aspirin tablet, but your aspirin will not be in a form that can be ingested because it will still be contaminated with harsh chemicals from the reaction. The reaction is given below; remember that each corner of the hexagon represents one carbon atom with enough hydrogen atoms to provide four bonds to each carbon atom. O O HO C O OH H3 C C O O C OH H3 C-C OH O + + H3 C-C H3 C-C O O salicylic acid + acetic anhydride acetylsalicylic acid + acetic acid Purpose: To synthesize acetylsalicylic acid on a microscale basis. Materials/Equipment: analytical balance filter paper stirring rod Hot plate microscale kit: test tube Hirsch funnel Filtering flasks Syringe (optional) salicylic acid acetic anhydride boiling chips conc. phosphoric acid Pure water 100 mL beaker thermometer vacuum pump ice/styrofoam cup Safety Considerations: Wear goggles and an apron at all times in the lab. Several of these chemicals will irritate your skin and eyes. If you spill anything on you, wash the area thoroughly with soap and water. Do not rub your face or eyes because you may get chemicals in your eyes. If your eyes start to burn, rinse them at the eye wash station. Do not ingest the aspirin you produce. It still contains harmful chemicals and is not fit to be taken. Procedure: 1. Accurately mass 135-140 mg of salicylic acid to the container. Record the mass (all four decimal places!) in the data table. Remember that the balances read “grams,” not “milligrams.” 2. Transfer all of the acid to a reaction tube (test tube) from the microscale kit. 3. Add one boiling chip and one drop of conc. phosphoric acid to the test tube. 4. Add 7 drops (approx. 0.3 mL) of acetic anhydride to the test tube. Try to rinse all the other ingredients to the bottom of the test tube when adding the acetic anhydride. 5. Shake the reaction tube vigorously side to side to mix the reactants thoroughly. 6. Fill a styrofoam cup about one-third full with hot water (between 70 and 90° C) from a central source. 7. Place the test tube in the water bath. You are trying to dissolve the salicylic acid and may need to agitate the test tube while it is in the water bath. 8. While waiting write your name in pencil on a piece of filter paper. Determine its mass using an analytical balance, and record it in the data table (all of the digits). 9. Once the acid is dissolved (contents will be completely clear), heat the solution in the reaction tube for 2 minutes longer. Cautiously add 12 drops (0.2 mL) of water to decompose excess acetic anhydride. 10. Remove the test tube from the water bath and allow it to cool to room temperature. If crystallization does not occur spontaneously at room temperature, use a spatula to scratch the inside of the test tube. Check with your teacher before doing this step. 11. Once crystallization has started, cool the test tube in a cup of ice water for several minutes until crystallization is complete. Be careful not to let the tube tip over and spill into the ice water. 12. Press the piece of filter paper in the Hirsch funnel. Transfer all of the contents of the test tube to the Hirsch funnel. You may use your spatula, rinsing it and the test tube with ice water, to be sure you transfer all of your product to the funnel. 13. Filter your product using the vacuum pump provided. 14. If a drying oven is available, dry the crystals on the filter paper thoroughly in the oven and let cool to room temperature before massing. Do not let the paper heat so long that it burns. If a drying oven is not available, see step 15. 15. If a drying oven is not available, it will be necessary to dry the crystals overnight. Write your name on a plastic bag with a Sharpie. Mass it using an analytical balance. Make sure that the bag does not touch the sides or bottom of the balance case. Record the mass in the data table (all of the digits). Place filter paper with product in the bag and allow to dry overnight. 16. To clean your lab station, rinse all glassware and the funnel and return them to the microscale kit. You may need to roll a paper towel carefully to wipe clean the inside of the reaction tube. 17. Once your product is dry, mass the filter paper with product. [If you dried the product in a plastic bag overnight, be sure to mass the filter paper with product in the plastic bag.] Record its mass in the table below. Then calculate the mass of the dry product. 18. It is common practice among synthetic organic chemists to calculate the percent yield of a product. You may also wish to check its purity by measuring the melting point with a Mel-Temp and comparing it to the accepted value. You may also wish to measure the melting point of the reactant in order to see that a chemical change actually occurred. Name(s) ____________________________________________Period ______Date_______________ Data: Mass of salicylic acid used Mass of filter paper [Mass of plastic bag, if bag was used] [Mass of bag, filter paper and product, if bag was used] Mass of filter paper and product Melting Points (optional) Salicylic acid (reactant) Acetylsalicylic acid (product) Calculations: (limiting reactant version) 1. You reacted salicylic acid and acetic anhydride to form aspirin, or acetylsalicylic acid. The reagent that runs out is called the limiting reagent because it limits the amount of product that can be formed. Determine which reactant, acetic anhydride or salicylic acid, will be used up first. The density of acetic anhydride is 1.08 g/mL. You used 7 drops of acetic anhydride (approximately 0.3 mL). HINT: Write a unit path to outline how you will calculate the quantities needed to determine the limiting reactant. 2. Calculate the theoretical yield, that is, the mass of aspirin that should be formed if all of the limiting reactant is used to form acetylsalicylic acid. 3. Calculate the percent yield for your reaction given actual amount of product formed (experimen tal) percent yield = x 100 theoretica l amount of product formed Name(s) ____________________________________________Period ______Date_______________ Data: Mass of salicylic acid used Mass of filter paper [Mass of plastic bag, if bag was used] [Mass of bag, filter paper and product, if bag was used] Mass of filter paper and product Melting Points (optional) Salicylic acid (reactant) Acetylsalicylic acid (product) Calculations (non-limiting reactant version): You reacted salicylic acid and acetic anhydride to form aspirin, or acetylsalicylic acid. You were directed to use an excess amount of the acetic anhydride so that the amount of aspirin produced would be determined by the amount of salicylic acid reacted. 1. Calculate the molar mass of salicylic acid, C7H6O3. 2. From the mass of salicylic acid measured out, calculate the moles of salicylic acid reacted. 3. From the equation in the introduction, determine the number of moles of acetylsalicylic acid produced by the moles of salicylic acid calculated above. 4. Calculate the mass of aspirin that should have been produced, the theoretical yield. (HINT: First calculate the molar mass of the acetylsalicylic acid.) 5. Calculate the percent yield for your reaction. actual amount of product formed (experimen tal) percent yield = x 100 theoretica l amount of product formed Name(s) ____________________________________________Period ______Date_______________ Questions: 1. What is aspirin? 2. How does aspirin work? 3. What are some of the side effects of aspirin? 4. What are the natural and synthetic sources of aspirin? 5. Who first made aspirin naturally and synthetically? 6. What is the chemical formula for aspirin? 7. If you got less than 100% yield, pose an explanation or two about how you might have lost some of your aspirin. 8. What chemicals may still be present that would contaminate your aspirin product? 9. How could you use a Mel-temp (a melting point apparatus) to check the purity of the aspirin you produced?