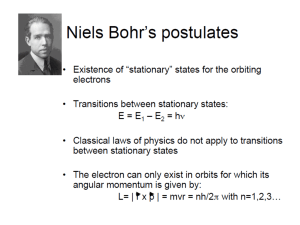
One of Bohr's postulates for hydrogen was
advertisement

One of Bohr's postulates for hydrogen was that the electron and the proton were stable in the atom only if some quantity had a minimum value or some integer times that minimum value. That quantity was a. b. c. d. energy. charge. angular momentum. radius. • If an electron in a hydrogen atom makes a transition from some state to a lower state do the following increase or decrease – Kinetic energy – Potential energy – Orbital angular momentum The approximate energy of the lowest Bohr orbit in the hydrogen atom is a. 0.1 eV b. 1.0 eV c. 10.0 eV d. 100 eV e. -100 eV f. -10.0 eV g. -1.0 eV h. -0.1 eV • What energy is required to ionize a hydrogen atom with an electron in the first excited state? (remove the electron from the atom?) Light: Particles or Waves? • • • • • • Newton (1675) Particle (travels in straight lines) Young (1801) Wave (Double Slit Experiment) Hertz (1880) Wave (Radio) Einstein (1905) Particle (Photoelectric effect) Laue (1911) Wave (X-Ray diffraction from crystals) Compton (1920) Particle (X-Ray scattering) Question: Who’s right? Less Filling? Tastes Great? Consider Young’s Double Slit Experiment Experiment. What would you change to make this experiment more profound? The famous Auto-Slit Experiment!