Equilibrium Problems
advertisement

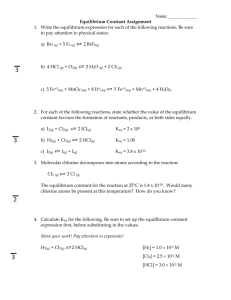
Equilibrium Problems Name_______________ Hr___ (ICE and No ICE) 1. At 427°C, a 3.00L flask contains 60.0mol H2, 54.0mol H2O, 36.0mol CO2 and 17.7mol CO at equilibrium. Calculate Keq for the reaction: CO2(g) + H2(g) <====> CO(g) + H2O(g) 2. Carbon monoxide reacts with steam to produce carbon dioxide and hydrogen (all gases). At 700K, the equilibrium constant is 5.10. Calculate the equilibrium concentrations of all compounds if 1.00mol of each reactant is mixed in a 1.00-L flask. 3. Hydrogen gas and chlorine gas react together and establish equilibrium with hydrogen chloride gas. The Keq for the synthesis reaction of HCl at 25°C is 4.00. At equilibrium the concentration of HCl is 0.600M. Assuming hydrogen and chlorine are present in equal amounts, calculate the concentration of hydrogen gas at equilibrium. 4. The Keq for the following reaction at room temperature is 1.70 x 102. If the equilibrium concentration of N2O4 is 2.30 x 10-1M, what is the equilibrium concentration of NO2? N2O4 <===> 2NO2 5. A 4.00-L flask was initially filled with 1.78mol of gaseous N2 and 3.56mol of gaseous H2 and heated to 400oC. After equilibrium was reached, 0.0400mol of gaseous NH3 was detected. Calculate Keq for this reaction. N2(g) + 3 H2(g) <===> 2 NH3(g) 6. Phosphorus pentachloride gas decomposes to gaseous phosphorus trichloride and chlorine gas at 25°C. Initially 4.00mol of phosphorus pentachloride in a 2.00L vessel are allowed to come to equilibrium. At equilibrium 1.80mol of chlorine gas is are detected. Calculate the concentrations of the other gases at equilibrium and the equilibrium constant. 7. At a particular temperature, a 1.00L flask contains 3.50mol HI, 4.10mol H2 and 0.300mol I2 at equilibrium. Calculate the Keq at this temperature for the reaction: H2(g) + I2(g) <====> 2HI(g)