The Nitrogen Cycle
advertisement

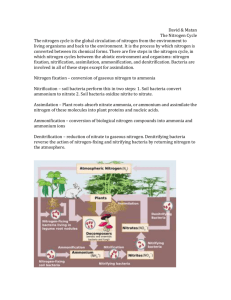
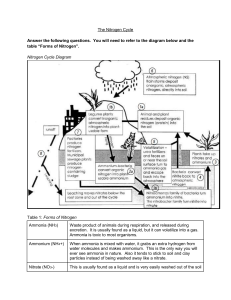
The Nitrogen Cycle The nitrogen cycle is the way that nitrogen in nature is changed into many different forms that are used by living [organism]s. Air is about 78% nitrogen. Nitrogen is needed for life. It is an important part of proteins, DNA, and RNA. In plants, nitrogen is needed for photosynthesis and growth. However, the nitrogen in the atmosphere is in its elemental form which is not usable by living things Nitrogen fixation is needed to change the nitrogen in air (N2) into reactive forms. Some reactive forms, like ammonium and nitrate, can be used by life. Most nitrogen fixation is done by micro-organisms called bacteria. Some of these bacteria live in the roots of plants, others live in soil or water. All nitrogen in animals comes from eating plants. 1. What is nitrogen fixation? Ammonium (NH4) in soil is made by nitrogen-fixing bacteria and decomposers, bacteria and fungi that break down dead life and animal waste into its parts. This process is called ammonification. Ammonium is also produced by fish and excreted directly into water in the form of fish waste. Ammonia and ammonium are poisonous to fish and other animals. Sewage and other waste-water is regularly measured because of this. If ammonia levels are too high, nitrification must happen. 2. Why is too much ammonium harmful? Nitrification is the change of ammonia and ammonium to nitrite (NO2−) and then to nitrate ( NO3−) by bacteria. High nitrate levels can also cause too much algae growth in lakes and pools. Algae are single-celled plants. They benefit from nitrate because nitrate is a form of nitrogen that plants can use. Too much algae can be harmful to fish and other water animals. The use of fertilizers containing ammonium is controlled more and more because of this. 3. What is nitrification? 4. Why can too much nitrate be harmful? 5. In the picture to the right, put the formulas for the following compounds in the correct places: Nitrate NO3Ammonium NH4+ Nitrite NO2-