The Nitrogen Cycle - Science 9 Portfolio
advertisement

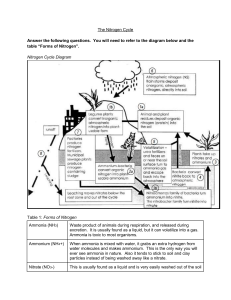
Jordan Bannerman The Nitrogen Cycle Answer the following questions. You will need to refer to the diagram below and the table “Forms of Nitrogen”. Nitrogen Cycle Diagram Table 1: Forms of Nitrogen Ammonia (NH3) Ammonium (NH4+) Nitrate (NO3-) Nitrite (NO2-) Nitrogen oxides (N2O and NOx) Organic Nitrogen (proteins) Nitric Acid (HNO3) Dinitrogen (N2) Waste product of animals during respiration, and released during excretion. It is usually found as a liquid, but it can volatilize into a gas. Ammonia is toxic to most organisms. When ammonia is mixed with water, it grabs an extra hydrogen from water molecules and makes ammonium. This is the only way you will ever see ammonia in nature. Also it tends to stick to soil and clay particles instead of being washed away like a nitrate. This is usually found as a liquid and is very easily washed out of the soil and into groundwater or streams. Farmers don’t like when their fertilizer gets turned into this because it washes away too easily and isn’t always in the soil long enough to help their plants. This is a middle step during the process of nitrification. It is very toxic to organisms. (In a new fish tank, nitrite can build up because the next step of nitrification can’t continue until all the ammonium is gone, so you have to carefully measure NO2- when you first add a fish to a tank) These are greenhouse gases, and a component of smog, and are a middle step (intermediate) between nitrate and N2 gas. Organic molecules containing nitrogen come in many forms from amino acids to proteins to lipids. This is the form of nitrogen in acid rain. It is also made by lightning. This is a gas and is one of the most abundant gases in the atmosphere Jordan Bannerman Note that the descriptions below correspond to the numbers shown in the Nitrogen Cycle Diagram. Fill in the blanks with the correct terms for each process or bacterial group described. 1. Animals deposit __Organic Nitrogen (Protein)___ into the soil. These chemicals are byproducts of their metabolism and respiration. Legume plants convert ______Inorganic atmospheric nitrogen_________ into plant-usable form is a process called _____Nitrogen fixing________. 2. a) ______Ammonium bacteria__________________ convert organic nitrogen into plant-usable ammonium. b) The ____Nitrosomonas_______ family of bacteria convert ammonium to nitrite. The _____Nitrobacter_________ family of bacteria convert nitrite to nitrate. 3. Plants take up ______Nitrates__________ and ________Ammonium_________. 4. What is it called when bacteria convert nitrate back to atmospheric nitrogen? _____Denitrofication______________________ 5. Urea fertilizers and feces on or near the soil surface turn to ammonia gas and escape back into the atmosphere. This process is called ______Volatization_____________. 6. Inorganic atmospheric nitrogen (N2) is deposited directly into the soil during ___Rain storms__________. 7. What human activity contributes to the accumulation of nitrogen in our soil and water? __Factories___ and _____Municipal Sewage________________________. 8. Runoff and ______Leaching_____________ moves nitrates below the “root zone and out of the cycle.